Role of Mitochondria in the Regulation of Effector Functions of Granulocytes
- PMID: 37759432
- PMCID: PMC10526294
- DOI: 10.3390/cells12182210
Role of Mitochondria in the Regulation of Effector Functions of Granulocytes
Abstract
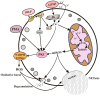
Granulocytes (neutrophils, eosinophils, and basophils) are the most abundant circulating cells in the innate immune system. Circulating granulocytes, primarily neutrophils, can cross the endothelial barrier and activate various effector mechanisms to combat invasive pathogens. Eosinophils and basophils also play an important role in allergic reactions and antiparasitic defense. Granulocytes also regulate the immune response, wound healing, and tissue repair by releasing of various cytokines and lipid mediators. The effector mechanisms of granulocytes include the production of reactive oxygen species (ROS), degranulation, phagocytosis, and the formation of DNA-containing extracellular traps. Although all granulocytes are primarily glycolytic and have only a small number of mitochondria, a growing body of evidence suggests that mitochondria are involved in all effector functions as well as in the production of cytokines and lipid mediators and in apoptosis. It has been shown that the production of mitochondrial ROS controls signaling pathways that mediate the activation of granulocytes by various stimuli. In this review, we will briefly discuss the data on the role of mitochondria in the regulation of effector and other functions of granulocytes.
Keywords: apoptosis; degranulation; extracellular traps; granulocytes; inflammation; leukotrienes; mitochondria; mitochondria-targeted antioxidants; mitochondrial ROS production; oxidative burst.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as potential conflict of interest.
Figures

Similar articles
-
Systemic and mucosal mobilization of granulocyte subsets during lentiviral infection.Immunology. 2021 Oct;164(2):348-357. doi: 10.1111/imm.13376. Epub 2021 Jun 16. Immunology. 2021. PMID: 34037988 Free PMC article.
-
Neutrophil and Eosinophil DNA Extracellular Trap Formation: Lessons From Pathogenic Fungi.Front Microbiol. 2021 Feb 18;12:634043. doi: 10.3389/fmicb.2021.634043. eCollection 2021. Front Microbiol. 2021. PMID: 33679665 Free PMC article. Review.
-
The Regulatory Effects of Interleukin-4 Receptor Signaling on Neutrophils in Type 2 Immune Responses.Front Immunol. 2019 Oct 24;10:2507. doi: 10.3389/fimmu.2019.02507. eCollection 2019. Front Immunol. 2019. PMID: 31708926 Free PMC article. Review.
-
"NETs and EETs, a Whole Web of Mess".Microorganisms. 2020 Dec 4;8(12):1925. doi: 10.3390/microorganisms8121925. Microorganisms. 2020. PMID: 33291570 Free PMC article. Review.
-
Functional characteristics of circulating granulocytes in severe congenital neutropenia caused by ELANE mutations.BMC Pediatr. 2019 Jun 8;19(1):189. doi: 10.1186/s12887-019-1556-x. BMC Pediatr. 2019. PMID: 31176364 Free PMC article.
Cited by
-
The causal relationship between thoracic aortic aneurysm and immune cells: a mendelian randomization study.BMC Cardiovasc Disord. 2024 Apr 16;24(1):212. doi: 10.1186/s12872-024-03876-1. BMC Cardiovasc Disord. 2024. PMID: 38627614 Free PMC article.
-
Multifaceted roles of mitochondria in asthma.Cell Biol Toxicol. 2024 Oct 9;40(1):85. doi: 10.1007/s10565-024-09928-8. Cell Biol Toxicol. 2024. PMID: 39382744 Free PMC article. Review.
-
Tafazzin regulates neutrophil maturation and inflammatory response.EMBO Rep. 2025 Mar;26(6):1590-1619. doi: 10.1038/s44319-025-00393-w. Epub 2025 Feb 17. EMBO Rep. 2025. PMID: 39962231 Free PMC article.
-
Identification of mitochondria-related feature genes for predicting type 2 diabetes mellitus using machine learning methods.Front Endocrinol (Lausanne). 2025 Mar 27;16:1501159. doi: 10.3389/fendo.2025.1501159. eCollection 2025. Front Endocrinol (Lausanne). 2025. PMID: 40213100 Free PMC article.
-
Role of immune dysregulation in peri-implantitis.Front Immunol. 2024 Nov 1;15:1466417. doi: 10.3389/fimmu.2024.1466417. eCollection 2024. Front Immunol. 2024. PMID: 39555067 Free PMC article. Review.
References
-
- Karnovsky M.L. The Metabolism of Leukocytes. Semin. Hematol. 1968;5:156–165. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

