New High-Affinity Thrombin Aptamers for Advancing Coagulation Therapy: Balancing Thrombin Inhibition for Clot Prevention and Effective Bleeding Management with Antidote
- PMID: 37759453
- PMCID: PMC10526462
- DOI: 10.3390/cells12182230
New High-Affinity Thrombin Aptamers for Advancing Coagulation Therapy: Balancing Thrombin Inhibition for Clot Prevention and Effective Bleeding Management with Antidote
Abstract
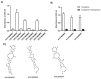
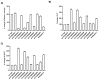
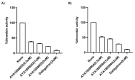
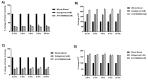
Thrombin is a key enzyme involved in blood clotting, and its dysregulation can lead to thrombotic diseases such as stroke, myocardial infarction, and deep vein thrombosis. Thrombin aptamers have the potential to be used as therapeutic agents to prevent or treat thrombotic diseases. Thrombin DNA aptamers developed in our laboratory exhibit high affinity and specificity to thrombin. In vitro assays have demonstrated their efficacy by significantly decreasing Factor II activity and increasing PT and APTT times in both plasma and whole blood. Aptamers AYA1809002 and AYA1809004, the two most potent aptamers, exhibit high affinity for their target, with affinity constants (Kd) of 10 nM and 13 nM, respectively. Furthermore, the in vitro activity of these aptamers displays dose-dependent behavior, highlighting their efficacy in a concentration-dependent manner. In vitro stability assessments reveal that the aptamers remain stable in plasma and whole blood for up to 24 h. This finding is crucial for their potential application in clinical settings. Importantly, the thrombin inhibitory activity of the aptamers can be reversed by employing reverse complement sequences, providing a mechanism to counteract their anticoagulant effects when necessary to avoid excessive bleeding. These thrombin aptamers have been determined to be safe, with no observed mutagenic or immunogenic effects. Overall, these findings highlight the promising characteristics of these newly developed thrombin DNA aptamers, emphasizing their potential for therapeutic applications in the field of anticoagulation therapy. Moreover, the inclusion of an antidote in the coagulation therapy regimen can improve patient safety, ensure greater therapeutic efficacy, and minimize risk during emergency situations.
Keywords: PE; VTE; aptamer; clot; coagulation cascade; thrombin; thrombosis.
Conflict of interest statement
The authors declare that they have no competing interest.
Figures







References
-
- World Thrombosis Day. 2023. [(accessed on 14 June 2023)]. Available online: https://www.worldthrombosisday.org/issue/thrombosis.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

