Size Exclusion Chromatography-Mass Photometry: A New Method for Adeno-Associated Virus Product Characterization
- PMID: 37759487
- PMCID: PMC10528216
- DOI: 10.3390/cells12182264
Size Exclusion Chromatography-Mass Photometry: A New Method for Adeno-Associated Virus Product Characterization
Abstract
Over the past decade, adeno-associated viruses (AAVs) have attained significant prominence in gene therapy and genome editing applications, necessitating the development of robust and precise methodologies to ensure the quality and purity of AAV products. Existing AAV characterization techniques have proven effective for the analysis of pure and homogeneous AAV samples. However, there is still a demand for a rapid and low-sample-consumption method suitable for the characterization of lower purity or heterogeneous AAV samples commonly encountered in AAV products. Addressing this challenge, we propose the SEC-MP method, which combines size exclusion chromatography (SEC) with mass photometry (MP). In this novel approach, SEC effectively separates monomeric AAV particles from impurities, while the UV detector determines the virus particle concentration. MP complements this process by estimating the fraction of fully packaged AAVs in the total population of AAV particles. This combined methodology enables accurate determination of the titer of effective, fully packaged AAVs in samples containing aggregates, incorrectly packaged AAVs with incomplete genomes, protein or DNA fragments, and other impurities. Our experimental results demonstrate that SEC-MP provides valuable guidance for sample quality control and subsequent applications in the field of AAV research.
Keywords: AAV titer; Vg/Cp ratio; gene therapy; sample quality.
Conflict of interest statement
The authors declare no financial or non-financial interests that are directly or indirectly related to the work submitted for publication.
Figures



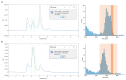
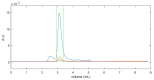

References
-
- NIH U.S. National Library of Medicine. [(accessed on 31 July 2023)]; Available online: https://clinicaltrials.gov/search?term=aav.
-
- FDA Chemistry, Manufacturing, and Control (CMC) Information for Human Gene Therapy Investigational New Drug Applications (INDs) [(accessed on 31 July 2023)]; Available online: https://www.fda.gov/regulatory-information/search-fda-guidance-documents....
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

