Modulation of Pro-Inflammatory IL-6 Trans-Signaling Axis by Splice Switching Oligonucleotides as a Therapeutic Modality in Inflammation
- PMID: 37759507
- PMCID: PMC10526877
- DOI: 10.3390/cells12182285
Modulation of Pro-Inflammatory IL-6 Trans-Signaling Axis by Splice Switching Oligonucleotides as a Therapeutic Modality in Inflammation
Abstract
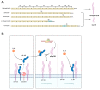
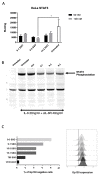
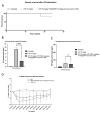
Interleukin-6 (IL-6) is a pleiotropic cytokine that plays a crucial role in maintaining normal homeostatic processes under the pathogenesis of various inflammatory and autoimmune diseases. This context-dependent effect from a cytokine is due to two distinctive forms of signaling: cis-signaling and trans-signaling. IL-6 cis-signaling involves binding IL-6 to the membrane-bound IL-6 receptor and Glycoprotein 130 (GP130) signal-transducing subunit. By contrast, in IL-6 trans-signaling, complexes of IL-6 and the soluble form of the IL-6 receptor (sIL-6R) signal via membrane-bound GP130. Various strategies have been employed in the past decade to target the pro-inflammatory effect of IL-6 in numerous inflammatory disorders. However, their development has been hindered since these approaches generally target global IL-6 signaling, also affecting the anti-inflammatory effects of IL-6 signaling too. Therefore, novel strategies explicitly targeting the pro-inflammatory IL-6 trans-signaling without affecting the IL-6 cis-signaling are required and carry immense therapeutic potential. Here, we have developed a novel approach to specifically decoy IL-6-mediated trans-signaling by modulating alternative splicing in GP130, an IL-6 signal transducer, by employing splice switching oligonucleotides (SSO), to induce the expression of truncated soluble isoforms of the protein GP130. This isoform is devoid of signaling domains but allows for specifically sequestering the IL-6/sIL-6R receptor complex with high affinity in serum and thereby suppressing inflammation. Using the state-of-the-art Pip6a cell-penetrating peptide conjugated to PMO-based SSO targeting GP130 for efficient in vivo delivery, reduced disease phenotypes in two different inflammatory mouse models of systemic and intestinal inflammation were observed. Overall, this novel gene therapy platform holds great potential as a refined therapeutic intervention for chronic inflammatory diseases.
Keywords: IL-6 trans-signaling; inflammation; nucleic acid therapies; oligonucleotides.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- Kamimura D., Arima Y., Hirano T., Ogura H., Murakami M. Cytokine Frontiers. Springer; Berlin/Heidelberg, Germany: 2014. - DOI
-
- Hirano T. Interleukin 6 in autoimmune and inflammatory diseases: A personal memoir. [(accessed on 19 May 2015)];Proc. Jpn. Acad. Ser. B Phys. Biol. Sci. 2010 86:717–730. doi: 10.2183/pjab.86.717. Available online: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=3066534&tool=p.... - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

