Unveiling the Secrets of the Stressed Hippocampus: Exploring Proteomic Changes and Neurobiology of Posttraumatic Stress Disorder
- PMID: 37759512
- PMCID: PMC10527244
- DOI: 10.3390/cells12182290
Unveiling the Secrets of the Stressed Hippocampus: Exploring Proteomic Changes and Neurobiology of Posttraumatic Stress Disorder
Abstract
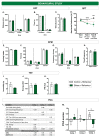
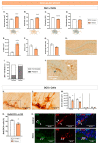
Intense stress, especially traumatic stress, can trigger disabling responses and in some cases even lead to the development of posttraumatic stress disorder (PTSD). PTSD is heterogeneous, accompanied by a range of distress symptoms and treatment-resistant disorders that may be associated with a number of other psychopathologies. PTSD is a very heterogeneous disorder with different subtypes that depend on, among other factors, the type of stressor that provokes it. However, the neurobiological mechanisms are poorly understood. The study of early stress responses may hint at the way PTSD develops and improve the understanding of the neurobiological mechanisms involved in its onset, opening the opportunity for possible preventive treatments. Proteomics is a promising strategy for characterizing these early mechanisms underlying the development of PTSD. The aim of the work was to understand how exposure to acute and intense stress using water immersion restraint stress (WIRS), which could be reminiscent of natural disaster, may induce several PTSD-associated symptoms and changes in the hippocampal proteomic profile. The results showed that exposure to WIRS induced behavioural symptoms and corticosterone levels reminiscent of PTSD. Moreover, the expression profiles of hippocampal proteins at 1 h and 24 h after stress were deregulated in favour of increased inflammation and reduced neuroplasticity, which was validated by histological studies and cytokine determination. Taken together, these results suggest that neuroplastic and inflammatory dysregulation may be a therapeutic target for the treatment of post-traumatic stress disorders.
Keywords: corticosterone; cytokines; hippocampal inflammation; hippocampus; natural disaster; neuroplasticity; posttraumatic stress disorder (PTSD); proteomic; water immersion restraint stress (WIRS).
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- American Psychiatric Association . Diagnostic and Statistical Manual of Mental Disorders (DSM-5) 5th ed. American Psychiatric Association; Washington, DC, USA: 2013.
-
- Staniaszek K., Cyniak-Cieciura M., Zawadzki B. Posttraumatic stress disorder symptom profiles—The role of temperament, traumatization, and cognitive factors. Pers. Individ. Differ. 2022;193:111595. doi: 10.1016/j.paid.2022.111595. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources

