Characterisation of Lipoma-Preferred Partner as a Novel Mechanotransducer in Vascular Smooth Muscle Cells
- PMID: 37759537
- PMCID: PMC10529303
- DOI: 10.3390/cells12182315
Characterisation of Lipoma-Preferred Partner as a Novel Mechanotransducer in Vascular Smooth Muscle Cells
Abstract
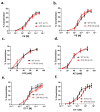
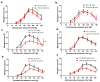
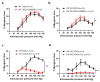
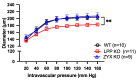
In arteries and arterioles, a chronic increase in blood pressure raises wall tension. This continuous biomechanical strain causes a change in gene expression in vascular smooth muscle cells (VSMCs) that may lead to pathological changes. Here we have characterised the functional properties of lipoma-preferred partner (LPP), a Lin11-Isl1-Mec3 (LIM)-domain protein, which is most closely related to the mechanotransducer zyxin but selectively expressed by smooth muscle cells, including VSMCs in adult mice. VSMCs isolated from the aorta of LPP knockout (LPP-KO) mice displayed a higher rate of proliferation than their wildtype (WT) counterparts, and when cultured as three-dimensional spheroids, they revealed a higher expression of the proliferation marker Ki 67 and showed greater invasion into a collagen gel. Accordingly, the gelatinase activity was increased in LPP-KO but not WT spheroids. The LPP-KO spheroids adhering to the collagen gel responded with decreased contraction to potassium chloride. The relaxation response to caffeine and norepinephrine was also smaller in the LPP-KO spheroids than in their WT counterparts. The overexpression of zyxin in LPP-KO VSMCs resulted in a reversal to a more quiescent differentiated phenotype. In native VSMCs, i.e., in isolated perfused segments of the mesenteric artery (MA), the contractile responses of LPP-KO segments to potassium chloride, phenylephrine or endothelin-1 did not vary from those in isolated perfused WT segments. In contrast, the myogenic response of LPP-KO MA segments was significantly attenuated while zyxin-deficient MA segments displayed a normal myogenic response. We propose that LPP, which we found to be expressed solely in the medial layer of different arteries from adult mice, may play an important role in controlling the quiescent contractile phenotype of VSMCs.
Keywords: LPP; VSMC; mechanosensitive genes; mechanotransduction; vascular remodelling.
Conflict of interest statement
All authors declare they have no financial interest to disclose that would be directly or indirectly related to the work submitted for publication.
Figures







References
-
- Owens G.K., Kumar M.S., Wamhoff B.R., Franco P.N., Durrant L.M., Carreon D., Haddad E., Vergara A., Cascavita C., Obenaus A., et al. Molecular Regulation of Vascular Smooth Muscle Cell Differentiation in Development and Disease. Physiol. Rev. 2004;84:767–801. doi: 10.1152/physrev.00041.2003. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

