A Story of Kinases and Adaptors: The Role of Lck, ZAP-70 and LAT in Switch Panel Governing T-Cell Development and Activation
- PMID: 37759563
- PMCID: PMC10525366
- DOI: 10.3390/biology12091163
A Story of Kinases and Adaptors: The Role of Lck, ZAP-70 and LAT in Switch Panel Governing T-Cell Development and Activation
Abstract
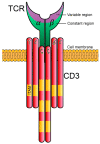
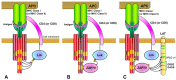
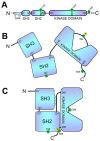
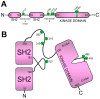
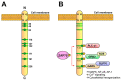
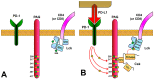
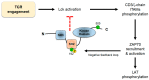
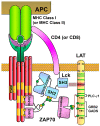
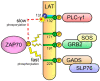
Specific antigen recognition is one of the immune system's features that allows it to mount intense yet controlled responses to an infinity of potential threats. T cells play a relevant role in the host defense and the clearance of pathogens by means of the specific recognition of peptide antigens presented by antigen-presenting cells (APCs), and, to do so, they are equipped with a clonally distributed antigen receptor called the T-cell receptor (TCR). Upon the specific engagement of the TCR, multiple intracellular signals are triggered, which lead to the activation, proliferation and differentiation of T lymphocytes into effector cells. In addition, this signaling cascade also operates during T-cell development, allowing for the generation of cells that can be helpful in the defense against threats, as well as preventing the generation of autoreactive cells. Early TCR signals include phosphorylation events in which the tyrosine kinases Lck and ZAP70 are involved. The sequential activation of these kinases leads to the phosphorylation of the transmembrane adaptor LAT, which constitutes a signaling hub for the generation of a signalosome, finally resulting in T-cell activation. These early signals play a relevant role in triggering the development, activation, proliferation and apoptosis of T cells, and the negative regulation of these signals is key to avoid aberrant processes that could generate inappropriate cellular responses and disease. In this review, we will examine and discuss the roles of the tyrosine kinases Lck and ZAP70 and the membrane adaptor LAT in these cellular processes.
Keywords: CD3; ITAMs; LAT; Lck; TCR; ZAP70; signaling.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses or interpretation of the data; in the writing of the manuscript; or in the decision to publish the results.
Figures
References
Publication types
Grants and funding
- PY20_01297/Consejería de Transformación Económica, Industria, Conocimiento y Universidades, Junta de Andalucía, Spain
- PID2020-113943RB-I00/Agencia Estatal de Investigación, Ministerio de Ciencia e Innovación, Spain
- PR2022-037/University of Cádiz
- PAIDI2020/DOC_01433/Consejería de Transformación Económica, Industria, Conocimiento y Universidades, Junta de Andalucía, Spain
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

