Head-to-Head Comparison of Tissue Factor-Dependent Procoagulant Potential of Small and Large Extracellular Vesicles in Healthy Subjects and in Patients with SARS-CoV-2 Infection
- PMID: 37759632
- PMCID: PMC10525820
- DOI: 10.3390/biology12091233
Head-to-Head Comparison of Tissue Factor-Dependent Procoagulant Potential of Small and Large Extracellular Vesicles in Healthy Subjects and in Patients with SARS-CoV-2 Infection
Abstract
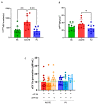
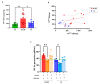
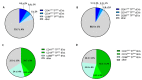
The relative contribution of small (sEVs) and large extracellular vesicles (lEVs) to the total plasma procoagulant potential is not yet well defined. Thus, we compared total and TFpos-sEVs and -lEVs isolated from healthy subjects and COVID-19 patients during the acute phase of the infection and after symptom remission in terms of (1) vesicle enumeration using nanoparticle tracking assay, imaging flow cytometry, and TF immunofluorescence localization in a single-vesicle analysis using microarrays; (2) cellular origin; and (3) TF-dependent Xa generation capacity, as well as assessing the contribution of the TF inhibitor, TFPI. In healthy subjects, the plasma concentration of CD9/CD63/CD81pos sEVs was 30 times greater than that of calceinpos lEVs, and both were mainly released by platelets. Compared to lEVs, the levels of TFpos-sEVs were 2-fold higher. The TF-dependent Xa generation capacity of lEVs was three times greater than that of sEVs, with the latter being hindered by TFPI. Compared to HSs, the amounts of total and TFpos-sEVs and -lEVs were significantly greater in acute COVID-19 patients, which reverted to the physiological values at the 6-month follow-up. Interestingly, the FXa generation of lEVs only significantly increased during acute infection, with that of sEV being similar to that of HSs. Thus, in both healthy subjects and COVID-19 patients, the TF-dependent procoagulant potential is mostly sustained by large vesicles.
Keywords: COVID-19; factor Xa generation; large extracellular vesicles; small extracellular vesicles; tissue factor.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Yates A.G., Pink R.C., Erdbrügger U., Siljander P.R.-M., Dellar E.R., Pantazi P., Akbar N., Cooke W.R., Vatish M., Dias-Neto E., et al. In sickness and in health: The functional role of extracellular vesicles in physiology and pathology in vivo: Part I: Health and Normal Physiology: Part I: Health and Normal Physiology. J. Extracell. Vesicles. 2022;11:e12151. doi: 10.1002/jev2.12151. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

