Functional Implications of Protein Arginine Methyltransferases (PRMTs) in Neurodegenerative Diseases
- PMID: 37759656
- PMCID: PMC10525691
- DOI: 10.3390/biology12091257
Functional Implications of Protein Arginine Methyltransferases (PRMTs) in Neurodegenerative Diseases
Abstract
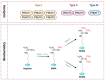
During the aging of the global population, the prevalence of neurodegenerative diseases will be continuously growing. Although each disorder is characterized by disease-specific protein accumulations, several common pathophysiological mechanisms encompassing both genetic and environmental factors have been detected. Among them, protein arginine methyltransferases (PRMTs), which catalyze the methylation of arginine of various substrates, have been revealed to regulate several cellular mechanisms, including neuronal cell survival and excitability, axonal transport, synaptic maturation, and myelination. Emerging evidence highlights their critical involvement in the pathophysiology of neurodegenerative diseases, including Alzheimer's disease (AD), Parkinson's disease (PD), frontotemporal dementia-amyotrophic lateral sclerosis (FTD-ALS) spectrum, Huntington's disease (HD), spinal muscular atrophy (SMA) and spinal and bulbar muscular atrophy (SBMA). Underlying mechanisms include the regulation of gene transcription and RNA splicing, as well as their implication in various signaling pathways related to oxidative stress responses, apoptosis, neuroinflammation, vacuole degeneration, abnormal protein accumulation and neurotransmission. The targeting of PRMTs is a therapeutic approach initially developed against various forms of cancer but currently presents a novel potential strategy for neurodegenerative diseases. In this review, we discuss the accumulating evidence on the role of PRMTs in the pathophysiology of neurodegenerative diseases, enlightening their pathogenesis and stimulating future research.
Keywords: Alzheimer’s disease; Huntington’s disease; PRMTs; Parkinson’s disease; amyotrophic lateral sclerosis; arginine methylation; neurodegeneration; spinal and bulbar muscular atrophy; spinal muscular atrophy.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
Publication types
LinkOut - more resources
Full Text Sources
Miscellaneous

