Advances in Dystrophinopathy Diagnosis and Therapy
- PMID: 37759719
- PMCID: PMC10526396
- DOI: 10.3390/biom13091319
Advances in Dystrophinopathy Diagnosis and Therapy
Abstract
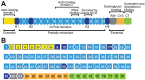
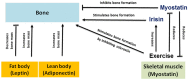
Dystrophinopathies are x-linked muscular disorders which emerge from mutations in the Dystrophin gene, including Duchenne and Becker muscular dystrophy, and dilated cardiomyopathy. However, Duchenne muscular dystrophy interconnects with bone loss and osteoporosis, which are exacerbated by glucocorticoids therapy. Procedures for diagnosing dystrophinopathies include creatine kinase assay, haplotype analysis, Southern blot analysis, immunological analysis, multiplex PCR, multiplex ligation-dependent probe amplification, Sanger DNA sequencing, and next generation DNA sequencing. Pharmacological therapy for dystrophinopathies comprises glucocorticoids (prednisone, prednisolone, and deflazacort), vamorolone, and ataluren. However, angiotensin-converting enzyme (ACE) inhibitors, angiotensin receptor blockers (ARBs), and β-blockers are the first-line to prevent dilated cardiomyopathy in dystrophinopathy patients. Duchenne muscular dystrophy gene therapy strategies involve gene transfer, exon skipping, exon reframing, and CRISPR gene editing. Eteplirsen, an antisense-oligonucleotide drug for skipping exon 51 from the Dystrophin gene, is available on the market, which may help up to 14% of Duchenne muscular dystrophy patients. There are various FDA-approved exon skipping drugs including ExonDys-51 for exon 51, VyonDys-53 and Viltolarsen for exon 53 and AmonDys-45 for exon 45 skipping. Other antisense oligonucleotide drugs in the pipeline include casimersen for exon 45, suvodirsen for exon 51, and golodirsen for exon 53 skipping. Advances in the diagnosis and therapy of dystrophinopathies offer new perspectives for their early discovery and care.
Keywords: Becker muscular dystrophy; CRISPR gene editing; Duchenne muscular dystrophy; dilated cardiomyopathy; dystrophinopathies; gene drugs; gene therapy; pharmacological therapy; prime gene editing; serum creatine kinase.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
-
- Monaco A.P., Bertelson C.J., Middlesworth W., Colletti C.A., Aldridge J., Fischbeck K.H., Bartlett R., Pericak-Vance M.A., Roses A.D., Kunkel L.M. Detection of deletions spanning the Duchenne muscular dystro-phy locus using a tightly linked DNA segment. Nature. 1985;316:842–845. doi: 10.1038/316842a0. - DOI - PubMed
-
- Koenig M., Hoffman E.P., Bertelson C.J., Monaco A.P., Feener C., Kunkel L.M. Complete cloning of the Duchenne muscular dystrophy (DMD) cDNA and preliminary genomic organization of the DMD gene in normal and affected individuals. Cell. 1987;50:509–517. doi: 10.1016/0092-8674(87)90504-6. - DOI - PubMed
-
- Gherardi S., Bovolenta M., Passarelli C., Falzarano M.S., Pigini P., Scotton C., Neri M., Armaroli A., Osman H., Selvatici R., et al. Transcriptional and epigenetic analyses of the DMD locus reveal novel cis acting DNA elements that govern muscle dystrophin expression. Biochim. Biophys. Acta Gene Regul. Mech. 2017;1860:1138–1147. doi: 10.1016/j.bbagrm.2017.08.010. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

