Low-Dose Chidamide Treatment Displays Sex-Specific Differences in the 3xTg-AD Mouse
- PMID: 37759724
- PMCID: PMC10526199
- DOI: 10.3390/biom13091324
Low-Dose Chidamide Treatment Displays Sex-Specific Differences in the 3xTg-AD Mouse
Abstract
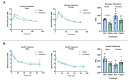
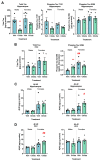
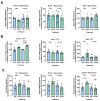
Epigenetic compounds have become attractive small molecules for targeting the multifaceted aspects of Alzheimer's disease (AD). Although AD disproportionately affects women, most of the current literature investigating epigenetic compounds for the treatment of AD do not report sex-specific results. This is remarkable because there is rising evidence that epigenetic compounds intrinsically affect males and females differently. This manuscript explores the sexual dimorphism observed after chronic, low-dose administration of a clinically relevant histone deacetylase inhibitor, chidamide (Tucidinostat), in the 3xTg-AD mouse model. We found that chidamide treatment significantly improves glucose tolerance and increases expression of glucose transporters in the brain of males. We also report a decrease in total tau in chidamide-treated mice. Differentially expressed genes in chidamide-treated mice were much greater in males than females. Genes involved in the neuroinflammatory pathway and amyloid processing pathway were mostly upregulated in chidamide-treated males while downregulated in chidamide-treated females. This work highlights the need for drug discovery projects to consider sex as a biological variable to facilitate translation.
Keywords: 3xTg-AD mouse; Alzheimer’s; HDAC inhibitor; epigenetics; sex differences.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Similar articles
-
Sex-Dependent Differences in Spontaneous Autoimmunity in Adult 3xTg-AD Mice.J Alzheimers Dis. 2018;63(3):1191-1205. doi: 10.3233/JAD-170779. J Alzheimers Dis. 2018. PMID: 29710702
-
Sexual Dimorphism in the 3xTg-AD Mouse Model and Its Impact on Pre-Clinical Research.J Alzheimers Dis. 2021;80(1):41-52. doi: 10.3233/JAD-201014. J Alzheimers Dis. 2021. PMID: 33459720 Free PMC article. Review.
-
Differential effects of chronic immunosuppression on behavioral, epigenetic, and Alzheimer's disease-associated markers in 3xTg-AD mice.Alzheimers Res Ther. 2021 Jan 20;13(1):30. doi: 10.1186/s13195-020-00745-9. Alzheimers Res Ther. 2021. PMID: 33472690 Free PMC article.
-
Role of sex and high-fat diet in metabolic and hypothalamic disturbances in the 3xTg-AD mouse model of Alzheimer's disease.J Neuroinflammation. 2020 Sep 29;17(1):285. doi: 10.1186/s12974-020-01956-5. J Neuroinflammation. 2020. PMID: 32993686 Free PMC article.
-
Chidamide: Targeting epigenetic regulation in the treatment of hematological malignancy.Hematol Oncol. 2023 Aug;41(3):301-309. doi: 10.1002/hon.3088. Epub 2022 Oct 25. Hematol Oncol. 2023. PMID: 36251458 Review.
Cited by
-
Low-dose dietary vorinostat increases brain histone acetylation levels and reduces oxidative stress in an Alzheimer's disease mouse model.J Alzheimers Dis. 2025 Jul 1;106(4):13872877251352107. doi: 10.1177/13872877251352107. Online ahead of print. J Alzheimers Dis. 2025. PMID: 40598871 Free PMC article.
-
Epigenetics-targeted drugs: current paradigms and future challenges.Signal Transduct Target Ther. 2024 Nov 26;9(1):332. doi: 10.1038/s41392-024-02039-0. Signal Transduct Target Ther. 2024. PMID: 39592582 Free PMC article. Review.
-
Epigenetic developmental mechanisms underlying sex differences in cancer.J Clin Invest. 2024 Jul 1;134(13):e180071. doi: 10.1172/JCI180071. J Clin Invest. 2024. PMID: 38949020 Free PMC article. Review.
References
-
- Volmar C.-H., Wahlestedt C. Histone Deacetylases (HDACs) and Brain Function. Neuroepigenetics. 2015;1:20–27. doi: 10.1016/j.nepig.2014.10.002. - DOI
-
- German Center for Neurodegenerative Diseases (DZNE) Multicenter, Open-Label Phase Ib Dose-Escalation and Dose-Confirmational Study for the Tolerability and Safety of N-Hydroxy-N’-Phenyl-Octanediamide (Vorinostat) in Patients With Mild Alzheimer’s Disease. ClinicalTrials.gov; Bethesda, MD, USA: 2019.
-
- Amylyx Pharmaceuticals Inc. Phase II Study to Assess the Safety, Tolerability, and Target Engagement of AMX0035, a Fixed Combination of Sodium Phenylbutyrate and Tauroursodeoxycholic Acid for the Treatment of Alzheimer’s Disease. ClinicalTrials.gov; Bethesda, MD, USA: 2021.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

