Infection, Inflammation, and Immunity in Sepsis
- PMID: 37759732
- PMCID: PMC10526286
- DOI: 10.3390/biom13091332
Infection, Inflammation, and Immunity in Sepsis
Abstract
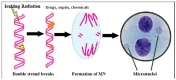
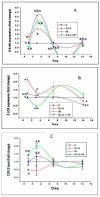
Sepsis is triggered by microbial infection, injury, or even major surgery. Both innate and adaptive immune systems are involved in its pathogenesis. Cytoplasmic presence of DNA or RNA of the invading organisms or damaged nuclear material (in the form of micronucleus in the cytoplasm) in the host cell need to be eliminated by various nucleases; failure to do so leads to the triggering of inflammation by the cellular cGAS-STING system, which induces the release of IL-6, TNF-α, and IFNs. These cytokines activate phospholipase A2 (PLA2), leading to the release of polyunsaturated fatty acids (PUFAs), gamma-linolenic acid (GLA), arachidonic acid (AA), eicosapentaenoic acid (EPA), and docosahexaenoic acid (DHA), which form precursors to various pro- and anti-inflammatory eicosanoids. On the other hand, corticosteroids inhibit PLA2 activity and, thus, suppress the release of GLA, AA, EPA, and DHA. PUFAs and their metabolites have a negative regulatory action on the cGAS-STING pathway and, thus, suppress the inflammatory process and initiate inflammation resolution. Pro-inflammatory cytokines and corticosteroids (corticosteroids > IL-6, TNF-α) suppress desaturases, which results in decreased formation of GLA, AA, and other PUFAs from the dietary essential fatty acids (EFAs). A deficiency of GLA, AA, EPA, and DHA results in decreased production of anti-inflammatory eicosanoids and failure to suppress the cGAS-STING system. This results in the continuation of the inflammatory process. Thus, altered concentrations of PUFAs and their metabolites, and failure to suppress the cGAS-STING system at an appropriate time, leads to the onset of sepsis. Similar abnormalities are also seen in radiation-induced inflammation. These results imply that timely administration of GLA, AA, EPA, and DHA, in combination with corticosteroids and anti-IL-6 and anti-TNF-α antibodies, may be of benefit in mitigating radiation-induced damage and sepsis.
Keywords: cGAS-STING system; cytokines; eicosanoids; inflammation; lipoxins; radiation; resolvins; sepsis; wound healing.
Conflict of interest statement
The author declares no conflict of interest.
Figures




References
-
- Singer M., Deutschman C.S., Seymour C.W., Shankar-Hari M., Annane D., Bauer M., Bellomo R., Bernard G.R., Chiche J.-D., Coopersmith C.M., et al. The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3) JAMA. 2016;315:801–810. doi: 10.1001/jama.2016.0287. - DOI - PMC - PubMed
-
- Monneret G. How to identify systemic sepsis-induced immunoparalysis. Adv. Sepsis. 2005;4:42–49.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

