AAV-Mediated Targeting of the Activin A-ACVR1R206H Signaling in Fibrodysplasia Ossificans Progressiva
- PMID: 37759764
- PMCID: PMC10526456
- DOI: 10.3390/biom13091364
AAV-Mediated Targeting of the Activin A-ACVR1R206H Signaling in Fibrodysplasia Ossificans Progressiva
Abstract
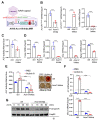
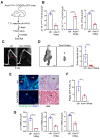
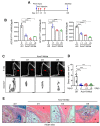
Fibrodysplasia ossificans progressiva (FOP) is an ultra-rare genetic disorder characterized by progressive disabling heterotopic ossification (HO) at extra-skeletal sites. Here, we developed adeno-associated virus (AAV)-based gene therapy that suppresses trauma-induced HO in FOP mice harboring a heterozygous allele of human ACVR1R206H (Acvr1R206H/+) while limiting the expression in non-skeletal organs such as the brain, heart, lung, liver, and kidney. AAV gene therapy carrying the combination of codon-optimized human ACVR1 (ACVR1opt) and artificial miRNAs targeting Activin A and its receptor ACVR1R206H ablated the aberrant activation of BMP-Smad1/5 signaling and the osteogenic differentiation of Acvr1R206H/+ skeletal progenitors. The local delivery of AAV gene therapy to HO-causing cells in the skeletal muscle resulted in a significant decrease in endochondral bone formation in Acvr1R206H/+ mice. These mice showed little to no expression in a major AAV-targeted organ, the liver, due to liver-abundant miR-122-mediated repression. Thus, AAV gene therapy is a promising therapeutic strategy to explore in suppressing HO in FOP.
Keywords: AAV; ACVR1; Activin A; fibrodysplasia ossificans progressiva; gene therapy; heterotopic ossificans.
Conflict of interest statement
G.G. and J.H.S. have submitted a patent application concerning the methodology described in this study. G.G. and J.H.S. are scientific co-founders of AAVAA Therapeutics and hold equity in this company. G.G. is also a scientific co-founder of Voyager Therapeutics and Aspa Therapeutics and holds equity in these companies. G.G. is an inventor on patents with potential royalties licensed to Voyager Therapeutics, Adrenas Therapeutics, Aspa Therapeutics Inc., and other biopharmaceutical companies. F.S.K. is the founder and past-President of the International Clinical Council (ICC) on FOP. F.S.K. serves in a volunteer capacity on the registry advisory board of the IFOPA. F.S.K. is an investigator on clinical trials sponsored by Clementia, an Ipsen company, and by Regeneron Pharmaceuticals.
Figures



References
-
- Pignolo R.J., Hsiao E.C., Baujat G., Lapidus D., Sherman A., Kaplan F.S. Prevalence of fibrodysplasia ossificans progressiva (FOP) in the United States: Estimate from three treatment centers and a patient organization. Orphanet J. Rare Dis. 2021;16:350. doi: 10.1186/s13023-021-01983-2. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

