Eicosapentaenoic Acid Influences the Lipid Profile of an In Vitro Psoriatic Skin Model Produced with T Cells
- PMID: 37759812
- PMCID: PMC10526348
- DOI: 10.3390/biom13091413
Eicosapentaenoic Acid Influences the Lipid Profile of an In Vitro Psoriatic Skin Model Produced with T Cells
Abstract
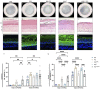
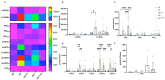
Psoriasis is a skin disease characterized by epidermal hyperplasia and an inappropriate activation of the adaptive immunity. A dysregulation of the skin's lipid mediators is reported in the disease with a predominance of the inflammatory cascade derived from n-6 polyunsaturated fatty acids (n-6 PUFAs). Bioactive lipid mediators derived from arachidonic acid (AA) are involved in the inflammatory functions of T cells in psoriasis, whereas n-3 PUFAs' derivatives are anti-inflammatory metabolites. Here, we sought to evaluate the influence of a supplementation of the culture media with eicosapentaenoic acid (EPA) on the lipid profile of a psoriatic skin model produced with polarized T cells. Healthy and psoriatic skin substitutes were produced following the auto-assembly technique. Psoriatic skin substitutes produced with or without T cells presented increased epidermal and dermal linolenic acid (LA) and AA levels. N-6 PUFA lipid mediators were strongly measured in psoriatic substitutes, namely, 13-hydroxyoctadecadienoic acid (13-HODE), prostaglandin E2 (PGE2) and 12-hydroxyeicosatetraenoic acid (12-HETE). The added EPA elevated the amounts of EPA, n-3 docosapentaenoic acid (DPA) and docosahexaenoic acid (DHA) in the epidermal and dermal phospholipids. The EPA supplementation balanced the production of epidermal lipid mediators, with an increase in prostaglandin E3 (PGE3), 12-hydroxyeicosapentaenoic acid (12-HEPE) and N-eicosapentaenoyl-ethanolamine (EPEA) levels. These findings show that EPA modulates the lipid composition of psoriatic skin substitutes by encouraging the return to a cutaneous homeostatic state.
Keywords: T cells; bioactive lipid mediators; n-3 PUFAs; psoriasis.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

