The Importance of M1-and M2-Polarized Macrophages in Glioma and as Potential Treatment Targets
- PMID: 37759870
- PMCID: PMC10526262
- DOI: 10.3390/brainsci13091269
The Importance of M1-and M2-Polarized Macrophages in Glioma and as Potential Treatment Targets
Abstract
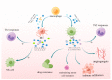
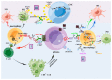
Glioma is the most common and malignant tumor of the central nervous system. Glioblastoma (GBM) is the most aggressive glioma, with a poor prognosis and no effective treatment because of its high invasiveness, metabolic rate, and heterogeneity. The tumor microenvironment (TME) contains many tumor-associated macrophages (TAMs), which play a critical role in tumor proliferation, invasion, metastasis, and angiogenesis and indirectly promote an immunosuppressive microenvironment. TAM is divided into tumor-suppressive M1-like (classic activation of macrophages) and tumor-supportive M2-like (alternatively activated macrophages) polarized cells. TAMs exhibit an M1-like phenotype in the initial stages of tumor progression, and along with the promotion of lysing tumors and the functions of T cells and NK cells, tumor growth is suppressed, and they rapidly transform into M2-like polarized macrophages, which promote tumor progression. In this review, we discuss the mechanism by which M1- and M2-polarized macrophages promote or inhibit the growth of glioblastoma and indicate the future directions for treatment.
Keywords: glioblastoma; glioma; polarization; treatment; tumor-associated macrophage.
Conflict of interest statement
The authors have no personal, financial, or institutional interests in the drugs, materials, or devices described in this article.
Figures


References
-
- Seyfrid M., Maich W.T., Shaikh V.M., Tatari N., Upreti D., Piyasena D., Subapanditha M., Savage N., McKenna D., Mikolajewicz N., et al. CD70 as an actionable immunotherapeutic target in recurrent glioblastoma and its microenvironment. J. Immunother. Cancer. 2022;10:e003289. doi: 10.1136/jitc-2021-003289. - DOI - PMC - PubMed
-
- Shi Y., Ping Y.F., Zhou W., He Z.C., Chen C., Bian B.S., Zhang L., Chen L., Lan X., Zhang X.C., et al. Tumour-associated macrophages secrete pleiotrophin to promote PTPRZ1 signalling in glioblastoma stem cells for tumour growth. Nat. Commun. 2017;8:15080. doi: 10.1038/ncomms15080. - DOI - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources

