The Cannabigerol Derivative VCE-003.2 Exerts Therapeutic Effects in 6-Hydroxydopamine-Lesioned Mice: Comparison with The Classic Dopaminergic Replacement Therapy
- PMID: 37759872
- PMCID: PMC10527302
- DOI: 10.3390/brainsci13091272
The Cannabigerol Derivative VCE-003.2 Exerts Therapeutic Effects in 6-Hydroxydopamine-Lesioned Mice: Comparison with The Classic Dopaminergic Replacement Therapy
Abstract
(1) Background: A cannabigerol aminoquinone derivative, so-called VCE-003.2, has been found to behave as a neuroprotective agent (administered both i.p. and orally) in different experimental models of Parkinson's disease (PD) in mice. These effects were exerted through mechanisms that involved the activation of a regulatory site within the peroxisome proliferator-activated receptor-γ (PPAR-γ). (2) Methods: We are now interested in comparing such neuroprotective potential of VCE-003.2, orally administered, with the effect of the classic dopaminergic replacement therapy with L-DOPA/benserazide in similar conditions, using 6-hydroxydopamine-lesioned mice. (3) Results: The oral administration of VCE-003.2 during 14 days at the dose of 20 mg/kg improved, as expected, the neurological status (measured in motor tests) in these mice. This correlated with a preservation of TH-labelled neurons in the substantia nigra. By contrast, the treatment with L-DOPA/benserazide (during 7 days at 2 mg/kg) was significantly less active in these experimental conditions, in concordance with their profile as a mere symptom-alleviating agent. (4) Conclusions: Our results confirmed again the therapeutic profile of VCE-003.2 in experimental PD and revealed a different and more relevant effect, as a disease modifier, compared to the classic symptom-alleviating L-DOPA treatment. This reinforces the interest in VCE-003.2 for a future clinical development in this disease.
Keywords: 6-OHDA-lesioned mice; L-DOPA/benserazide; PPAR-γ; Parkinson’s disease; VCE-003.2; cannabinoids.
Conflict of interest statement
The authors declare that they have no conflict of interest in relation to this study. The funders had no role in study design, the collection, analysis, and interpretation of data, in the writing of the report, or in the decision to submit the paper for publication.
Figures
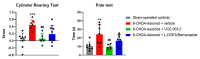
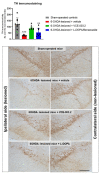
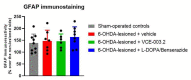

References
-
- Antonazzo M., Botta M., Bengoetxea H., Ruiz-Ortega J.A., Morera-Herreras T. Therapeutic potential of cannabinoids as neuroprotective agents for damaged cells conducing to movement disorders. Int. Rev. Neurobiol. 2019;146:229–257. - PubMed
LinkOut - more resources
Full Text Sources

