Repetitive, but Not Single, Mild Blast TBI Causes Persistent Neurological Impairments and Selective Cortical Neuronal Loss in Rats
- PMID: 37759899
- PMCID: PMC10526452
- DOI: 10.3390/brainsci13091298
Repetitive, but Not Single, Mild Blast TBI Causes Persistent Neurological Impairments and Selective Cortical Neuronal Loss in Rats
Abstract
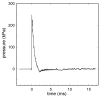
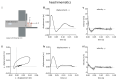
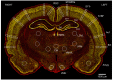
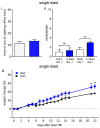
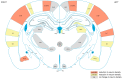
Exposure to repeated mild blast traumatic brain injury (mbTBI) is common in combat soldiers and the training of Special Forces. Evidence suggests that repeated exposure to a mild or subthreshold blast can cause serious and long-lasting impairments, but the mechanisms causing these symptoms are unclear. In this study, we characterise the effects of single and tightly coupled repeated mbTBI in Sprague-Dawley rats exposed to shockwaves generated using a shock tube. The primary outcomes are functional neurologic function (unconsciousness, neuroscore, weight loss, and RotaRod performance) and neuronal density in brain regions associated with sensorimotor function. Exposure to a single shockwave does not result in functional impairments or histologic injury, which is consistent with a mild or subthreshold injury. In contrast, exposure to three tightly coupled shockwaves results in unconsciousness, along with persistent neurologic impairments. Significant neuronal loss following repeated blast was observed in the motor cortex, somatosensory cortex, auditory cortex, and amygdala. Neuronal loss was not accompanied by changes in astrocyte reactivity. Our study identifies specific brain regions particularly sensitive to repeated mbTBI. The reasons for this sensitivity may include exposure to less attenuated shockwaves or proximity to tissue density transitions, and this merits further investigation. Our novel model will be useful in elucidating the mechanisms of sensitisation to injury, the temporal window of sensitivity and the evaluation of new treatments.
Keywords: blast neurotrauma; blast trauma; blast traumatic brain injury; concussion; functional deficits; repetitive brain injury.
Conflict of interest statement
Dickinson received funding as detailed above and acknowledges the financial support of the Royal British Legion. Rita Campos-Pires was the recipient of a doctoral training award from the Fundação para a Ciência e a Tecnologia, Lisbon, Portugal, and received funding from the Association of Paediatric Anaesthetists of Great Britain and Ireland. Mariia Koziakova was the recipient of a Rector’s PhD Studentship from Imperial College London. Eszter Ujvari is the recipient of an MRC PhD studentship. The other authors declare no competing interests.
Figures





References
-
- Maas A.I.R., Menon D.K., Adelson P.D., Andelic N., Bell M.J., Belli A., Bragge P., Brazinova A., Buki A., Chesnut R.M., et al. Traumatic brain injury: Integrated approaches to improve prevention, clinical care, and research. Lancet Neurol. 2017;16:987–1048. doi: 10.1016/S1474-4422(17)30371-X. - DOI - PubMed
-
- DoD_Worldwide_Numbers_for_TBI. [(accessed on 31 July 2023)]. Available online: https://dvbic.dcoe.mil/dod-worldwide-numbers-tbi.
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

