Nitric Oxide Induces Autophagy in Triticum aestivum Roots
- PMID: 37759958
- PMCID: PMC10525912
- DOI: 10.3390/antiox12091655
Nitric Oxide Induces Autophagy in Triticum aestivum Roots
Abstract
Autophagy is a highly conserved process that degrades damaged macromolecules and organelles. Unlike animals, only scant information is available regarding nitric oxide (NO)-induced autophagy in plants. Such lack of information prompted us to study the roles of the NO donors' nitrate, nitrite, and sodium nitroprusside in this catabolic process in wheat roots. Furthermore, spermine, a polyamine that is found in all eukaryotic cells, was also tested as a physiological NO donor. Here, we show that in wheat roots, NO donors and spermine can trigger autophagy, with NO and reactive oxygen species (ROS) playing signaling roles based on the visualization of autophagosomes, analyses of the levels of NO, ROS, mitochondrial activity, and the expression of autophagic (ATG) genes. Treatment with nitrite and nitroprusside causes an energy deficit, a typical prerequisite of autophagy, which is indicated by a fall in mitochondrial potential, and the activity of mitochondrial complexes. On the contrary, spermine sustains energy metabolism by upregulating the activity of appropriate genes, including those that encode glyceraldehyde 3-phosphate dehydrogenase GAPDH and SNF1-related protein kinase 1 SnRK1. Taken together, our data suggest that one of the key roles for NO in plants may be to trigger autophagy via diverse mechanisms, thus facilitating the removal of oxidized and damaged cellular constituencies.
Keywords: autophagy; energy metabolism; nitric oxide; plant; spermine.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Figures

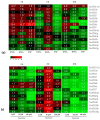


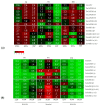
References
-
- He C. Mechanisms of autophagy: The machinery of macroautophagy and points of control. In: Rothermel B.A., Diwan A., editors. Autophagy in Health and Disease. 2nd ed. Elsevier; Philadelphia, PA, USA: Academic Press; Cambridge, MA, USA: 2022. pp. 9–19. - DOI
-
- Corpas F.J., Barroso J.B., Carreras A., Valderrama R., Palma J.M., León A.M., Sandalio L.M., del Río L.A. Constitutive arginine-dependent nitric oxide synthase activity in different organs of pea seedlings during plant development. Planta. 2006;224:246–254. doi: 10.1007/s00425-005-0205-9. - DOI - PubMed
-
- Campbell W.H. Nitrate reductase and its role in nitrate assimilation in plants. Physiol. Plant. 1988;74:214–219. doi: 10.1111/j.1399-3054.1988.tb04965.x. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

