Changes in pH and Nitrite Nitrogen Induces an Imbalance in the Oxidative Defenses of the Spotted Babylon (Babylonia areolata)
- PMID: 37759962
- PMCID: PMC10526028
- DOI: 10.3390/antiox12091659
Changes in pH and Nitrite Nitrogen Induces an Imbalance in the Oxidative Defenses of the Spotted Babylon (Babylonia areolata)
Abstract
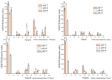
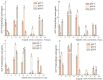
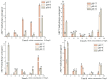
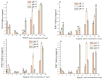
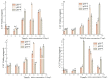
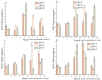
In order to reveal the acute toxicity and physiological changes of the spotted babylon (Babylonia areolata) in response to environmental manipulation, the spotted babylon was exposed to three pH levels (7.0, 8.0 and 9.0) of seawater and four concentrations of nitrite nitrogen (0.02, 2.7, 13.5 and 27 mg/L). The activities of six immunoenzymes, superoxide dismutase (SOD), glutathione peroxidase (GSH-PX), catalase (CAT), acid phosphatase (ACP), alkaline phosphatase (AKP) and peroxidase (POD), were measured. The levels of pH and nitrite nitrogen concentrations significantly impacted immunoenzyme activity over time. After the acute stress of pH and nitrite nitrogen, the spotted babylon appeared to be unresponsive to external stimuli, exhibited decreased vigor, slowly climbed the wall, sank to the tank and could not stand upright. As time elapsed, with the extension of time, the spotted babylon showed a trend of increasing and then decreasing ACP, AKP, CAT and SOD activities in order to adapt to the mutated environment and improve its immunity. In contrast, POD and GSH-PX activities showed a decrease followed by an increase with time. This study explored the tolerance range of the spotted babylon to pH, nitrite nitrogen, and time, proving that external stimuli activate the body's immune response. The body's immune function has a specific range of adaptation to the environment over time. Once the body's immune system was insufficient to adapt to this range, the immune system collapsed and the snail gradually died off. This study has discovered the suitable pH and nitrite nitrogen ranges for the culture of the spotted babylon, and provides useful information on the response of the snail's immune system.
Keywords: acidity; alkalinity; behavior; gastrapods; immunase; nitrous acid.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Similar articles
-
Response of antioxidation and immunity to combined influences of pH and ammonia nitrogen in the spotted babylon (Babylonia areolata).Heliyon. 2024 Apr 5;10(8):e29205. doi: 10.1016/j.heliyon.2024.e29205. eCollection 2024 Apr 30. Heliyon. 2024. PMID: 38638986 Free PMC article.
-
Post-mortem changes in farmed spotted Babylon snail (Babylonia areolata) during iced storage.Food Sci Technol Int. 2010 Jun;16(3):277-84. doi: 10.1177/1082013209353830. Epub 2010 Aug 12. Food Sci Technol Int. 2010. PMID: 21339144
-
The antioxidant potential of peptides obtained from the spotted babylon snail (Babylonia areolata) in treating human colon adenocarcinoma (Caco-2) cells.RSC Adv. 2020 Jul 7;10(43):25746-25757. doi: 10.1039/d0ra03261a. eCollection 2020 Jul 3. RSC Adv. 2020. PMID: 35518590 Free PMC article.
-
Corrigendum to 'The presence of abalone egg-laying hormone-like peptide in the central nervous system and ovary of the Spotted Babylon, Babylonia areolata' [Acta histochemica 119 (2017) 701-707].Acta Histochem. 2018 Nov;120(8):859. doi: 10.1016/j.acthis.2018.11.003. Acta Histochem. 2018. PMID: 30446259 No abstract available.
-
The presence of abalone egg-laying hormone-like peptide in the central nervous system and ovary of the Spotted Babylon, Babylonia areolata.Acta Histochem. 2017 Sep;119(7):701-707. doi: 10.1016/j.acthis.2017.08.006. Epub 2017 Sep 14. Acta Histochem. 2017. PMID: 28919178
Cited by
-
Effect of intestinal microbiota on growth rate of Babylonia areolata.PLoS One. 2025 May 7;20(5):e0322985. doi: 10.1371/journal.pone.0322985. eCollection 2025. PLoS One. 2025. PMID: 40334216 Free PMC article.
References
-
- Zhou J.C., Liu C., Yang Y.M., Yang Y., Gu Z.F., Wang A.M., Liu C.S. Effects of long-term exposure to ammonia on growth performance, immune response, and body biochemical composition of juvenile ivory shell, Babylonia. Aquaculture. 2023;562:738857.
-
- Gregory T.D., Nguyen D.Q.D., Nicholas A.P., Paul C., Southgate P.C. Assessing potential for integrating sea grape (Caulerpa lentillifera) culture with sandfish (Holothuria scabra) and Babylon snail (Babylonia) co-culture. Aquaculture. 2020;522:735153.
-
- Lü W., Zhong M., Fu J., Ke S., Gan B., Zhou Y., Shen M., Ke C. Comparison and Optimal Prediction of Goptimal prediction of growth of Babylonia areolata and B. lutosa. Aquac. Rep. 2020;18:100425.
-
- Chen C.-Z., Li P., Liu L., Li Z.-H. Exploring the interactions between the gut microbiome and the shifting surrounding aquatic environment in fisheries and aquaculture: A review. Environ. Res. 2022;214:114202. - PubMed
Grants and funding
- ZDYF2022XDNY351/Hainan Province Science and Technology Special Fund
- 2022YJ10, 2020TD55/Central Public-interest Scientific Institution Basal Research Fund, CAFS
- 202201010054/the earmarked fund for CARS (CARS-49), Science and Technology Planning Project of Guangzhou
- NHYYSWZZZYKZX2020/Financial Fund of Ministry of Agriculture and Rural affairs of China
- 2019YFD0900800/National Key R&D Program of China
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

