Artifacts Introduced by Sample Handling in Chemiluminescence Assays of Nitric Oxide Metabolites
- PMID: 37759975
- PMCID: PMC10525973
- DOI: 10.3390/antiox12091672
Artifacts Introduced by Sample Handling in Chemiluminescence Assays of Nitric Oxide Metabolites
Abstract
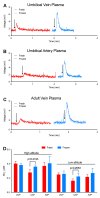
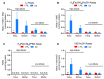
We recently developed a combination of four chemiluminescence-based assays for selective detection of different nitric oxide (NO) metabolites, including nitrite, S-nitrosothiols (SNOs), heme-nitrosyl (heme-NO), and dinitrosyl iron complexes (DNICs). However, these NO species (NOx) may be under dynamic equilibria during sample handling, which affects the final determination made from the readout of assays. Using fetal and maternal sheep from low and high altitudes (300 and 3801 m, respectively) as models of different NOx levels and compositions, we tested the hypothesis that sample handling introduces artifacts in chemiluminescence assays of NOx. Here, we demonstrate the following: (1) room temperature placement is associated with an increase and decrease in NOx in plasma and whole blood samples, respectively; (2) snap freezing and thawing lead to the interconversion of different NOx in plasma; (3) snap freezing and homogenization in liquid nitrogen eliminate a significant fraction of NOx in the aorta of stressed animals; (4) A "stop solution" commonly used to preserve nitrite and SNOs leads to the interconversion of different NOx in blood, while deproteinization results in a significant increase in detectable NOx; (5) some reagents widely used in sample pretreatments, such as mercury chloride, acid sulfanilamide, N-ethylmaleimide, ferricyanide, and anticoagulant ethylenediaminetetraacetic acid, have unintended effects that destabilize SNO, DNICs, and/or heme-NO; (6) blood, including the residual blood clot left in the washed purge vessel, quenches the signal of nitrite when using ascorbic acid and acetic acid as the purge vessel reagent; and (7) new limitations to the four chemiluminescence-based assays. This study points out the need for re-evaluation of previous chemiluminescence measurements of NOx, and calls for special attention to be paid to sample handling, as it can introduce significant artifacts into NOx assays.
Keywords: DNIC; NO metabolites; Pitfall; S-nitrosothiol; chemiluminescence methodology; heme-NO; nitrite.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
Grants and funding
LinkOut - more resources
Full Text Sources

