Pharmacologic Ascorbate and DNMT Inhibitors Increase DUOX Expression and Peroxide-Mediated Toxicity in Pancreatic Cancer
- PMID: 37759986
- PMCID: PMC10525653
- DOI: 10.3390/antiox12091683
Pharmacologic Ascorbate and DNMT Inhibitors Increase DUOX Expression and Peroxide-Mediated Toxicity in Pancreatic Cancer
Abstract
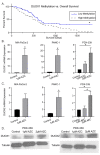
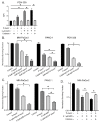
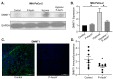
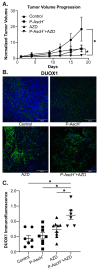
Recent studies have demonstrated an important role for vitamin C in the epigenetic regulation of cancer-related genes via DNA demethylation by the ten-eleven translocation (TET) methylcytosine dioxygenase enzymes. DNA methyltransferase (DNMT) reverses this, increasing DNA methylation and decreasing gene expression. Dual oxidase (DUOX) enzymes produce hydrogen peroxide (H2O2) in normal pancreatic tissue but are silenced in pancreatic cancer (PDAC). Treatment of PDAC with pharmacologic ascorbate (P-AscH-, intravenous, high dose vitamin C) increases DUOX expression. We hypothesized that inhibiting DNMT may act synergistically with P-AscH- to further increase DUOX expression and cytotoxicity of PDAC. PDAC cells demonstrated dose-dependent increases in DUOX mRNA and protein expression when treated with DNMT inhibitors. PDAC cells treated with P-AscH- + DNMT inhibitors demonstrated increased DUOX expression, increased intracellular oxidation, and increased cytotoxicity in vitro and in vivo compared to either treatment alone. These findings suggest a potential therapeutic, epigenetic mechanism to treat PDAC.
Keywords: DNA methyltransferase (DNMT); ascorbic acid; epigenetics; pancreatic cancer; pharmacologic ascorbate; ten-eleven translocation (TET) methylcytosine dioxygenase.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Similar articles
-
Dual Oxidase-Induced Sustained Generation of Hydrogen Peroxide Contributes to Pharmacologic Ascorbate-Induced Cytotoxicity.Cancer Res. 2020 Apr 1;80(7):1401-1413. doi: 10.1158/0008-5472.CAN-19-3094. Epub 2020 Feb 10. Cancer Res. 2020. PMID: 32041838 Free PMC article.
-
Pharmacologic ascorbate (P-AscH-) suppresses hypoxia-inducible Factor-1α (HIF-1α) in pancreatic adenocarcinoma.Clin Exp Metastasis. 2018 Feb;35(1-2):37-51. doi: 10.1007/s10585-018-9876-z. Epub 2018 Feb 2. Clin Exp Metastasis. 2018. PMID: 29396728 Free PMC article.
-
Ascorbate induces ten-eleven translocation (Tet) methylcytosine dioxygenase-mediated generation of 5-hydroxymethylcytosine.J Biol Chem. 2013 May 10;288(19):13669-74. doi: 10.1074/jbc.C113.464800. Epub 2013 Apr 2. J Biol Chem. 2013. PMID: 23548903 Free PMC article.
-
Local chromatin microenvironment determines DNMT activity: from DNA methyltransferase to DNA demethylase or DNA dehydroxymethylase.Epigenetics. 2015;10(8):671-6. doi: 10.1080/15592294.2015.1062204. Epigenetics. 2015. PMID: 26098813 Free PMC article. Review.
-
Auranofin and Pharmacologic Ascorbate as Radiomodulators in the Treatment of Pancreatic Cancer.Antioxidants (Basel). 2022 May 14;11(5):971. doi: 10.3390/antiox11050971. Antioxidants (Basel). 2022. PMID: 35624835 Free PMC article. Review.
Cited by
-
Endoscopic surgery affects the gut microbiota and its metabolism in breast cancer patients.Front Microbiol. 2025 Jan 7;15:1481582. doi: 10.3389/fmicb.2024.1481582. eCollection 2024. Front Microbiol. 2025. PMID: 39839115 Free PMC article.
-
The therapeutic potential of vitamins A, C, and D in pancreatic cancer.Heliyon. 2024 Dec 31;11(1):e41598. doi: 10.1016/j.heliyon.2024.e41598. eCollection 2025 Jan 15. Heliyon. 2024. PMID: 39850424 Free PMC article. Review.
-
Pharmacological ascorbate combined with rucosopasem selectively radio-chemo-sensitizes NSCLC via generation of H2O2.Redox Biol. 2025 Mar;80:103505. doi: 10.1016/j.redox.2025.103505. Epub 2025 Jan 23. Redox Biol. 2025. PMID: 39884000 Free PMC article.
References
-
- Goldstein D., El-Maraghi R.H., Hammel P., Heinemann V., Kunzmann V., Sastre J., Scheithauer W., Siena S., Tabernero J., Teixeira L., et al. nab-Paclitaxel plus gemcitabine for metastatic pancreatic cancer: Long-term survival from a phase III trial. J. Natl. Cancer Inst. 2015;107:dju413. doi: 10.1093/jnci/dju413. - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

