Exogenous Melatonin Protects against Oxidative Damage to Membrane Lipids Caused by Some Sodium/Iodide Symporter Inhibitors in the Thyroid
- PMID: 37759991
- PMCID: PMC10525497
- DOI: 10.3390/antiox12091688
Exogenous Melatonin Protects against Oxidative Damage to Membrane Lipids Caused by Some Sodium/Iodide Symporter Inhibitors in the Thyroid
Abstract
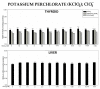
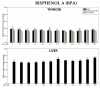
The thyroid gland is the primary site of sodium/iodide symporter (NIS), an intrinsic plasma membrane protein responsible for the active uptake of iodine, which is indispensable for thyroid hormone synthesis. Since exposure of the thyroid to NIS inhibitors can potentially have harmful effects on the entire organism, it is important to investigate the potential protective effects of known antioxidants, such as melatonin and indole-3-propionic acid (IPA), against pro-oxidative action of classic NIS inhibitors. The study aimed to check if and to what extent melatonin and IPA interact with some confirmed NIS inhibitors regarding their effects on oxidative damage to membrane lipids in the thyroid. For comparison with the thyroid gland, in which NIS is typically present, the liver tissue-not possessing NIS-was applied in the present study. Thyroid and liver homogenates were incubated in the presence of tested NIS inhibitors (i.e., NaClO3, NH4SCN, KSeCN, KNO3, NaF, KClO4, and BPA) in different ranges of concentrations with/without melatonin (5 mM) or IPA (5 mM). The malondialdehyde+4-hydroxyalkenals (MDA + 4-HDA) concentration (LPO index) was measured spectrophotometrically. NaClO3 increased LPO in the thyroid and in the liver, but these pro-oxidative effects were not prevented by either melatonin or IPA. Instead, pro-oxidative effects of NH4SCN observed in both tissues were prevented by both indole substances. KSeCN and NaF increased LPO only in the thyroid, and these pro-oxidative effects were prevented by melatonin and IPA. KNO3, KClO4, and BPA did not increase LPO, which can be due to their low concentrations resulting from restricted solubility. In conclusion, as melatonin prevented oxidative damage to membrane lipids in the thyroid caused by some sodium/iodide symporter inhibitors, this indoleamine shoud be considered as a potential protective agent when produced appropriately in living organisms but also as an exogenous substance recommended to individuals overexposed to NIS inhibitors.
Keywords: lipid peroxidation; melatonin; sodium/iodide symporter inhibitors; thyroid.
Conflict of interest statement
The authors declare no conflict of interest.
Figures






References
-
- USEPA . Inorganic Chlorates Facts. US Environmental Protection Agency; Washington, DC, USA: 2008. pp. 1–6. EPA 738-F-08-001.
Grants and funding
LinkOut - more resources
Full Text Sources

