Reactive Oxygen Species and H. pylori Infection: A Comprehensive Review of Their Roles in Gastric Cancer Development
- PMID: 37760015
- PMCID: PMC10525271
- DOI: 10.3390/antiox12091712
Reactive Oxygen Species and H. pylori Infection: A Comprehensive Review of Their Roles in Gastric Cancer Development
Abstract
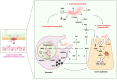
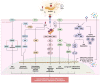
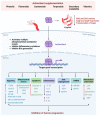
Gastric cancer (GC) is the fifth most common cancer worldwide and makes up a significant component of the global cancer burden. Helicobacter pylori (H. pylori) is the most influential risk factor for GC, with the International Agency for Research on Cancer classifying it as a Class I carcinogen for GC. H. pylori has been shown to persist in stomach acid for decades, causing damage to the stomach's mucosal lining, altering gastric hormone release patterns, and potentially altering gastric function. Epidemiological studies have shown that eliminating H. pylori reduces metachronous cancer. Evidence shows that various molecular alterations are present in gastric cancer and precancerous lesions associated with an H. pylori infection. However, although H. pylori can cause oxidative stress-induced gastric cancer, with antioxidants potentially being a treatment for GC, the exact mechanism underlying GC etiology is not fully understood. This review provides an overview of recent research exploring the pathophysiology of H. pylori-induced oxidative stress that can cause cancer and the antioxidant supplements that can reduce or even eliminate GC occurrence.
Keywords: Helicobacter pylori; antioxidants; gastric cancer; inflammation; oxidative stress.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Yang L., Kartsonaki C., Yao P., de Martel C., Plummer M., Chapman D., Guo Y., Clark S., Walters R.G., Chen Y. The relative and attributable risks of cardia and non-cardia gastric cancer associated with Helicobacter pylori infection in China: A case-cohort study. Lancet Public Health. 2021;6:e888–e896. doi: 10.1016/S2468-2667(21)00164-X. - DOI - PMC - PubMed
-
- Wang Z., Dai J., Hu N., Miao X., Abnet C.C., Yang M., Freedman N.D., Chen J., Burdette L., Zhu X. Identification of new susceptibility loci for gastric non-cardia adenocarcinoma: Pooled results from two Chinese genome-wide association studies. Gut. 2017;66:581–587. doi: 10.1136/gutjnl-2015-310612. - DOI - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Miscellaneous

