NRF2, a Superstar of Ferroptosis
- PMID: 37760042
- PMCID: PMC10525540
- DOI: 10.3390/antiox12091739
NRF2, a Superstar of Ferroptosis
Abstract
Ferroptosis is an iron-dependent and lipid peroxidation-driven cell death cascade, occurring when there is an imbalance of redox homeostasis in the cell. Nuclear factor erythroid 2-related factor 2 (NFE2L2, also known as NRF2) is key for cellular antioxidant responses, which promotes downstream genes transcription by binding to their antioxidant response elements (AREs). Numerous studies suggest that NRF2 assumes an extremely important role in the regulation of ferroptosis, for its various functions in iron, lipid, and amino acid metabolism, and so on. Many pathological states are relevant to ferroptosis. Abnormal suppression of ferroptosis is found in many cases of cancer, promoting their progression and metastasis. While during tissue damages, ferroptosis is recurrently promoted, resulting in a large number of cell deaths and even dysfunctions of the corresponding organs. Therefore, targeting NRF2-related signaling pathways, to induce or inhibit ferroptosis, has become a great potential therapy for combating cancers, as well as preventing neurodegenerative and ischemic diseases. In this review, a brief overview of the research process of ferroptosis over the past decade will be presented. In particular, the mechanisms of ferroptosis and a focus on the regulation of ferroptosis by NRF2 will be discussed. Finally, the review will briefly list some clinical applications of targeting the NRF2 signaling pathway in the treatment of diseases.
Keywords: NRF2; antioxidant; ferroptosis; metabolism.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
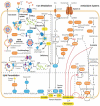
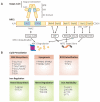
References
-
- Yagoda N., von Rechenberg M., Zaganjor E., Bauer A.J., Yang W.S., Fridman D.J., Wolpaw A.J., Smukste I., Peltier J.M., Boniface J.J., et al. RAS-RAF-MEK-dependent oxidative cell death involving voltage-dependent anion channels. Nature. 2007;447:864–868. doi: 10.1038/nature05859. - DOI - PMC - PubMed
-
- Friedmann Angeli J.P., Schneider M., Proneth B., Tyurina Y.Y., Tyurin V.A., Hammond V.J., Herbach N., Aichler M., Walch A., Eggenhofer E., et al. Inactivation of the ferroptosis regulator Gpx4 triggers acute renal failure in mice. Nat. Cell Biol. 2014;16:1180–1191. doi: 10.1038/ncb3064. - DOI - PMC - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

