Exploring the Potential of Olive Flounder Processing By-Products as a Source of Functional Ingredients for Muscle Enhancement
- PMID: 37760060
- PMCID: PMC10526038
- DOI: 10.3390/antiox12091755
Exploring the Potential of Olive Flounder Processing By-Products as a Source of Functional Ingredients for Muscle Enhancement
Abstract
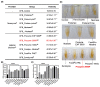
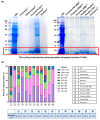
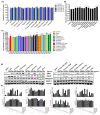
Olive flounder (OF) is a widely aqua-cultivated and recognized socioeconomic resource in Korea. However, more than 50% of by-products are generated when processing one OF, and there is no proper way to utilize them. With rising awareness and interest in eco-friendly bio-materialization recycling, this research investigates the potential of enzymatic hydrolysis of OF by-products (OFB) to produce functional ingredients. Various enzymatic hydrolysates of OFB (OFBEs) were generated using 11 commercial enzymes. Among them, Prozyme 2000P-assisted OFBE (OFBP) exhibited the highest protein content and yield, as well as low molecularization. The muscle regenerative potential of OFBEs was evaluated using C2C12 myoblasts, revealing that OFBP positively regulated myoblast differentiation. In an in vitro Dex-induced myotube atrophy model, OFBP protected against muscle atrophy and restored myotube differentiation and Dex-induced reactive oxygen species (ROS) production. Furthermore, zebrafish treated with OFBEs showed improved locomotor activity and body weight, with OFBP exhibiting outstanding restoration in the Dex-induced muscle atrophy zebrafish in vivo model. In conclusion, OFBEs, particularly OFBP, produce hydrolysates with enhanced physiological usability and muscle regenerative potential. Further research on its industrial application and mechanistic insights is needed to realize its potential as a high-quality protein food ingredient derived from OF processing by-products.
Keywords: dexamethasone-induced ROS production; dexamethasone-induced muscle atrophy; enzymatic hydrolysis; fish by-product; myogenesis; zebrafish.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




Similar articles
-
Olive Flounder By-Product Prozyme2000P Hydrolysate Ameliorates Age-Related Kidney Decline by Inhibiting Ferroptosis.Int J Mol Sci. 2024 Apr 25;25(9):4668. doi: 10.3390/ijms25094668. Int J Mol Sci. 2024. PMID: 38731887 Free PMC article.
-
Valorization of fishery byproducts: Large-scale production of olive flounder functional protein ingredients and their effects on muscle regeneration.Food Res Int. 2025 Mar;204:115931. doi: 10.1016/j.foodres.2025.115931. Epub 2025 Feb 5. Food Res Int. 2025. PMID: 39986777
-
Protein Hydrolysate from Spirulina platensis Prevents Dexamethasone-Induced Muscle Atrophy via Akt/Foxo3 Signaling in C2C12 Myotubes.Mar Drugs. 2022 May 29;20(6):365. doi: 10.3390/md20060365. Mar Drugs. 2022. PMID: 35736168 Free PMC article.
-
Dexamethasone Treatment at the Myoblast Stage Enhanced C2C12 Myocyte Differentiation.Int J Med Sci. 2017 Apr 8;14(5):434-443. doi: 10.7150/ijms.18427. eCollection 2017. Int J Med Sci. 2017. PMID: 28539819 Free PMC article.
-
Assessment of the biological activity of fish muscle protein hydrolysates using in vitro model systems.Food Chem. 2021 Oct 15;359:129852. doi: 10.1016/j.foodchem.2021.129852. Epub 2021 Apr 20. Food Chem. 2021. PMID: 33940471 Review.
Cited by
-
Alcalase-Assisted Mytilus edulis Hydrolysate: A Nutritional Approach for Recovery from Muscle Atrophy.Mar Drugs. 2023 Nov 29;21(12):623. doi: 10.3390/md21120623. Mar Drugs. 2023. PMID: 38132945 Free PMC article.
-
Targeting Aging Skin with GABALAGEN®: A Synergistic Marine Nutricosmetic Ingredient Validated Through Human Randomized Trials.Antioxidants (Basel). 2025 Feb 20;14(3):245. doi: 10.3390/antiox14030245. Antioxidants (Basel). 2025. PMID: 40227205 Free PMC article.
-
Exploring the Potential of Crassostrea nippona Hydrolysates as Dietary Supplements for Mitigating Dexamethasone-Induced Muscle Atrophy in C2C12 Cells.Mar Drugs. 2024 Feb 28;22(3):113. doi: 10.3390/md22030113. Mar Drugs. 2024. PMID: 38535454 Free PMC article.
-
Olive Flounder By-Product Prozyme2000P Hydrolysate Ameliorates Age-Related Kidney Decline by Inhibiting Ferroptosis.Int J Mol Sci. 2024 Apr 25;25(9):4668. doi: 10.3390/ijms25094668. Int J Mol Sci. 2024. PMID: 38731887 Free PMC article.
References
-
- Jeong N., Kim K. Nutrition knowledge and eating behaviors of elementary school children in Seoul. Korean J. Community Nutr. 2009;14:55–66.
-
- Jayawardhana H.H.A.C.K., Oh J.Y., Jayawardena T.U., Sanjeewa K.K.A., Liyanage N.M., Nagahawatta D.P., Hyun J., Son K.-T., Jeon Y.-J., Park J. Protective Effect of Fish Gut Hydrolysates from Olive Flounder (Paralichthys olivaceus) Surimi Byproducts Against AAPH-Induced Oxidative Stress in In Vitro and In Vivo Zebrafish Models. J. Aquat. Food Prod. Technol. 2022;31:924–938. doi: 10.1080/10498850.2022.2119909. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

