Palmitic Acid Upregulates CD96 Expression to Mediate Maternal-Foetal Interface Immune Tolerance by Inhibiting Cytotoxic Activity and Promoting Adhesion Function in Human Decidual Natural Killer Cells
- PMID: 37760110
- PMCID: PMC10525720
- DOI: 10.3390/bioengineering10091008
Palmitic Acid Upregulates CD96 Expression to Mediate Maternal-Foetal Interface Immune Tolerance by Inhibiting Cytotoxic Activity and Promoting Adhesion Function in Human Decidual Natural Killer Cells
Abstract
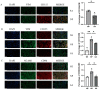
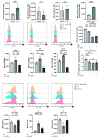
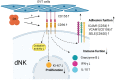
Decidual natural killer cells (dNK cells) are an essential component of the immune cells present at the maternal-foetal interface during early pregnancy, and they play a vital role in various physiological processes. Abnormalities in the ratio or function of dNK cells have been linked to recurrent miscarriages. CD96 has been previously shown to regulate NK cell function in the tumour microenvironment; however, its role and mechanism at the maternal-foetal interface remains unclear. The present study aimed to investigate the immunomodulatory role of CD96 in dNK cells and its function at the maternal-foetal interface. Immunofluorescence staining and flow cytometry were used to detect the expression of cellular markers such as CD96. Furthermore, the secretory function, adhesion-function-related molecules, and cell proliferation markers of CD96+ and CD96- dNK cells were detected using flow cytometry. In addition, we performed cell culture experiments via the magnetic bead sorting of NK cells to detect changes in the expression of the aforementioned functional molecules in dNK cells after the CD96 blockade. Furthermore, we examined the functional characteristics of dNK cells after palmitic acid treatment at a concentration of 10 μM. We also examined the changes in dNK cell function when subjected to the combined effect of palmitic acid and CD96 antagonists. The results indicated that CD96, TIGIT, CD155, and CD112 were highly expressed at the maternal-foetal interface, with dNK cells predominantly expressing CD96, whereas TIGIT was mainly expressed on T cells, and CD155 and CD112 were mainly present in metaphase stromal and trophoblast cells. CD96+ dNK cells displayed low cytotoxic activity and a high adhesion phenotype, which mediated the immunosuppressive effect on dNK cells at the maternal-foetal interface. Palmitic acid upregulated CD96 expression on the surface of dNK cells in the coculture system, inhibiting dNK cell activity and increasing their adhesion molecule expression. CD96 antagonist treatment blocked the inhibitory effect of trophoblasts on dNK cells, resulting in enhanced cytokine secretion and reduced adhesion. The results of this study provide valuable insight into the immunomodulatory role of CD96 in dNK cells and its mechanism at the maternal-foetal interface, particularly in metaphase NK cells. This study sheds light on the mechanisms of immune regulation at the maternal-foetal interface and their implications for the study of recurrent miscarriages of unknown origin.
Keywords: CD96; dNK cells; immune adhesion; maternal–foetal immunology; recurrent spontaneous abortion.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




Similar articles
-
Pigment epithelium-derived factor, a novel decidual natural killer cells-derived factor, protects decidual stromal cells via anti-inflammation and anti-apoptosis in early pregnancy.Hum Reprod. 2020 Jul 1;35(7):1537-1552. doi: 10.1093/humrep/deaa118. Hum Reprod. 2020. PMID: 32544239
-
Decidual CXCR4+ CD56bright NK cells as a novel NK subset in maternal-foetal immune tolerance to alleviate early pregnancy failure.Clin Transl Med. 2021 Oct;11(10):e540. doi: 10.1002/ctm2.540. Clin Transl Med. 2021. PMID: 34709764 Free PMC article.
-
Glycodelin-A stimulates the conversion of human peripheral blood CD16-CD56bright NK cell to a decidual NK cell-like phenotype.Hum Reprod. 2019 Apr 1;34(4):689-701. doi: 10.1093/humrep/dey378. Hum Reprod. 2019. PMID: 30597092
-
Killer Timing: The Temporal Uterine Natural Killer Cell Differentiation Pathway and Implications for Female Reproductive Health.Front Endocrinol (Lausanne). 2022 Jun 27;13:904744. doi: 10.3389/fendo.2022.904744. eCollection 2022. Front Endocrinol (Lausanne). 2022. PMID: 35832424 Free PMC article. Review.
-
The human decidual NK-cell response to virus infection: what can we learn from circulating NK lymphocytes?J Reprod Immunol. 2011 Mar;88(2):170-5. doi: 10.1016/j.jri.2010.12.005. Epub 2011 Jan 28. J Reprod Immunol. 2011. PMID: 21277025 Review.
Cited by
-
Dual Role of Natural Killer Cells in Early Pregnancy: Immunopathological Implications and Therapeutic Potential in Recurrent Spontaneous Abortion and Recurrent Implantation Failure.Cell Prolif. 2025 Sep;58(9):e70037. doi: 10.1111/cpr.70037. Epub 2025 May 5. Cell Prolif. 2025. PMID: 40325291 Free PMC article. Review.
-
Immunometabolism of ferroptosis in the tumor microenvironment.Front Oncol. 2024 Aug 12;14:1441338. doi: 10.3389/fonc.2024.1441338. eCollection 2024. Front Oncol. 2024. PMID: 39188677 Free PMC article. Review.
References
-
- Jing M., Chen X., Qiu H., He W., Zhou Y., Li D., Wang D., Jiao Y., Liu A. Insights into the immunomodulatory regulation of matrix metalloproteinase at the maternal-fetal interface during early pregnancy and pregnancy-related diseases. Front. Immunol. 2023;13:1067661. doi: 10.3389/fimmu.2022.1067661. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Research Materials

