The Development of a Permanent Implantable Spacer with the Function of Size Adjustability for Customized Treatment of Regurgitant Heart Valve Disease
- PMID: 37760118
- PMCID: PMC10525886
- DOI: 10.3390/bioengineering10091016
The Development of a Permanent Implantable Spacer with the Function of Size Adjustability for Customized Treatment of Regurgitant Heart Valve Disease
Abstract
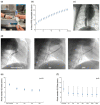
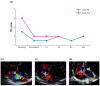
The Pivot Mandu is an innovative device featuring a leak-tight adjustable 3D balloon spacer, incorporating inner mesh support, an outer e-PTFE layer, and a compliant balloon in the middle layer with a specialized detachable system. To assess its feasibility, proof of concept was rigorously evaluated through bench testing and survival porcine animal experiments. The results demonstrated successful remote inflation of the balloon system, with the balloon spacer exhibiting sustained patent and functional integrity over an extended observation period of up to 6 months. A noteworthy feature of the newly designed 3D balloon spacer is its capability for easy size adjustment during procedures, enhancing its adaptability and practicality in clinical settings. This three-layered 3D balloon spacer, with its established long-term patency, exhibits highly encouraging outcomes that hold promise in overcoming the current limitations of spacer devices for heart valve diseases. Given the compelling results from preclinical investigations, the translation of the Pivot Mandu into human trials is strongly warranted.
Keywords: biocompatible coating; cardiology; e-PTFE; implantable medical device; nitinol; polyurethane.
Conflict of interest statement
The corresponding author (June-Hong Kim) has intellectual property of the spacer device and stock of TAU MEDICAL Inc., and is currently working as clinical director of Tau-PNU MEDICAL Co., Ltd. The other authors declare no conflict of interest.
Figures



References
-
- Thourani V.H., Kodali S., Makkar R.R., Herrmann H.C., Williams M., Babaliaros V., Smalling R., Lim S., Malaisrie S.C., Kapadia S. Transcatheter aortic valve replacement versus surgical valve replacement in intermediate-risk patients: A propensity score analysis. Lancet. 2016;387:2218–2225. doi: 10.1016/S0140-6736(16)30073-3. - DOI - PubMed
-
- Kim J.-H., Sung S.-C., Chon M.-K., Kim J.-O., Lee S.-H., Lee S.-Y., Je H.-G., Choo K.-S., Hwang J.-M., Kim J.-S. Mitral loop cerclage as a variant form of mitral cerclage annuloplasty that adds a device (CSTV) for preventing potential complications: A preclinical proof of concept and feasibility study. Eurointerv. J. Eur. Collab. Work. Group Interv. Cardiol. Eur. Soc. Cardiol. 2016;11:e1669–e1679. doi: 10.4244/EIJV11I14A319. - DOI - PubMed
-
- Asmarats L., Perlman G., Praz F., Hensey M., Chrissoheris M.P., Philippon F., Ofek H., Ye J., Puri R., Pibarot P. Long-term outcomes of the FORMA transcatheter tricuspid valve repair system for the treatment of severe tricuspid regurgitation: Insights from the first-in-human experience. JACC Cardiovasc. Interv. 2019;12:1438–1447. doi: 10.1016/j.jcin.2019.04.038. - DOI - PubMed
-
- Peppas A., Furer A., Wilson J., Yi G., Cheng Y., Van Wygerden K., Seguin C., Shibuya M., Kaluza G.L., Granada J.F. Preclinical in vivo long-term evaluation of the novel Mitra-Spacer technology: Experimental validation in the ovine model. Eurointerv. J. Eur. Collab. Work. Group Interv. Cardiol. Eur. Soc. Cardiol. 2017;13:272–279. doi: 10.4244/EIJ-D-16-00609. - DOI - PubMed
LinkOut - more resources
Full Text Sources

