Self-Reporting Theranostic: Nano Tool for Arterial Thrombosis
- PMID: 37760122
- PMCID: PMC10525380
- DOI: 10.3390/bioengineering10091020
Self-Reporting Theranostic: Nano Tool for Arterial Thrombosis
Abstract
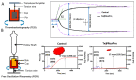
Arterial thrombosis (AT) originates through platelet-mediated thrombus formation in the blood vessel and can lead to heart attack, stroke, and peripheral vascular diseases. Restricting the thrombus growth and its simultaneous monitoring by visualisation is an unmet clinical need for a better AT prognosis. As a proof-of-concept, we have engineered a nanoparticle-based theranostic (combined therapy and monitoring) platform that has the potential to monitor and restrain the growth of a thrombus concurrently. The theranostic nanotool is fabricated using biocompatible super-paramagnetic iron oxide nanoparticles (SPIONs) as a core module tethered with the anti-platelet agent Abciximab (ReoPro) on its surface. Our in vitro feasibility results indicate that ReoPro-conjugated SPIONS (Tx@ReoPro) can effectively prevent thrombus growth by inhibiting fibrinogen receptors (GPIIbIIIa) on the platelet surface, and simultaneously, it can also be visible through non-invasive magnetic resonance imaging (MRI) for potential reporting of the real-time thrombus status.
Keywords: MRI; ReoPro; arterial thrombosis; platelet aggregations; theranostics.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



Similar articles
-
H2O2-triggered "off/on signal" nanoparticles target P-selectin for the non-invasive and contrast-enhanced theranostics for arterial thrombosis.Acta Biomater. 2023 Mar 1;158:769-781. doi: 10.1016/j.actbio.2022.12.026. Epub 2022 Dec 21. Acta Biomater. 2023. PMID: 36565786
-
Administration of abciximab during percutaneous coronary intervention reduces both ex vivo platelet thrombus formation and fibrin deposition: implications for a potential anticoagulant effect of abciximab.Arterioscler Thromb Vasc Biol. 1998 Aug;18(8):1342-9. doi: 10.1161/01.atv.18.8.1342. Arterioscler Thromb Vasc Biol. 1998. PMID: 9714143 Clinical Trial.
-
Theranostic MUC-1 aptamer targeted gold coated superparamagnetic iron oxide nanoparticles for magnetic resonance imaging and photothermal therapy of colon cancer.Colloids Surf B Biointerfaces. 2016 Jul 1;143:224-232. doi: 10.1016/j.colsurfb.2016.02.058. Epub 2016 Feb 27. Colloids Surf B Biointerfaces. 2016. PMID: 27015647
-
Targeted superparamagnetic iron oxide nanoparticles for early detection of cancer: Possibilities and challenges.Nanomedicine. 2016 Feb;12(2):287-307. doi: 10.1016/j.nano.2015.10.019. Epub 2015 Dec 18. Nanomedicine. 2016. PMID: 26707817 Review.
-
Recent advances in superparamagnetic iron oxide nanoparticles (SPIONs) for in vitro and in vivo cancer nanotheranostics.Int J Pharm. 2015 Dec 30;496(2):191-218. doi: 10.1016/j.ijpharm.2015.10.058. Epub 2015 Oct 28. Int J Pharm. 2015. PMID: 26520409 Review.
References
LinkOut - more resources
Full Text Sources
Research Materials

