Integrated Microfluidic-Electromagnetic System to Probe Single-Cell Magnetotaxis in Microconfinement
- PMID: 37760136
- PMCID: PMC10525280
- DOI: 10.3390/bioengineering10091034
Integrated Microfluidic-Electromagnetic System to Probe Single-Cell Magnetotaxis in Microconfinement
Abstract
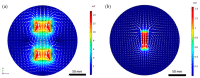
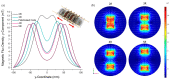
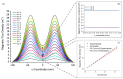
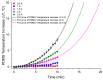
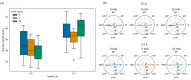
Magnetotactic bacteria have great potential for use in biomedical and environmental applications due to the ability to direct their navigation with a magnetic field. Applying and accurately controlling a magnetic field within a microscopic region during bacterial magnetotaxis studies at the single-cell level is challenging due to bulky microscope components and the inherent curvilinear field lines produced by commonly used bar magnets. In this paper, a system that integrates microfluidics and electromagnetic coils is presented for generating a linear magnetic field within a microenvironment compatible with microfluidics, enabling magnetotaxis analysis of groups or single microorganisms on-chip. The platform, designed and optimised via finite element analysis, is integrated into an inverted fluorescent microscope, enabling visualisation of bacteria at the single-cell level in microfluidic devices. The electromagnetic coils produce a linear magnetic field throughout a central volume where the microfluidic device containing the magnetotactic bacteria is located. The magnetic field, at this central position, can be accurately controlled from 1 to 10 mT, which is suitable for directing the navigation of magnetotactic bacteria. Potential heating of the microfluidic device from the operating coils was evaluated up to 2.5 A, corresponding to a magnetic field of 7.8 mT, for 10 min. The maximum measured heating was 8.4 °C, which enables analysis without altering the magnetotaxis behaviour or the average swimming speed of the bacteria. Altogether, this work provides a design, characterisation and experimental test of an integrated platform that enables the study of individual bacteria confined in microfluidics, under linear and predictable magnetic fields that can be easily and accurately applied and controlled.
Keywords: bacterial taxis; magnetotactic bacteria; magnetotaxis; microfluidics; microswimmer; single-cell analysis.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Frankel R.B., Williams T.J., Bazylinski D.A. Magneto-Aerotaxis. In: Schüler D., editor. Magnetoreception and Magnetosomes in Bacteria. Springer; Berlin/Heidelberg, Germany: 2006. pp. 1–24. - DOI
-
- Marcano L., Muñoz D., Martín-Rodríguez R., Orue I., Alonso J., García-Prieto A., Serrano A., Valencia S., Abrudan R., Barquín L.F., et al. Magnetic Study of Co-Doped Magnetosome Chains. J. Phys. Chem. C. 2018;122:7541–7550. doi: 10.1021/acs.jpcc.8b01187. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources

