Retention of Human iPSC-Derived or Primary Cells Following Xenotransplantation into Rat Immune-Privileged Sites
- PMID: 37760151
- PMCID: PMC10525500
- DOI: 10.3390/bioengineering10091049
Retention of Human iPSC-Derived or Primary Cells Following Xenotransplantation into Rat Immune-Privileged Sites
Abstract
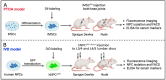
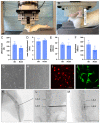
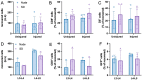
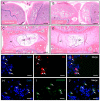
In regenerative medicine, experimental animal models are commonly used to study potential effects of human cells as therapeutic candidates. Although some studies describe certain cells, such as mesenchymal stromal cells (MSC) or human primary cells, as hypoimmunogenic and therefore unable to trigger strong inflammatory host responses, other studies report antibody formation and immune rejection following xenotransplantation. Accordingly, the goal of our study was to test the cellular retention and survival of human-induced pluripotent stem cell (iPSCs)-derived MSCs (iMSCs) and primary nucleus pulposus cells (NPCs) following their xenotransplantation into immune-privileged knee joints (14 days) and intervertebral discs (IVD; 7 days) of immunocompromised Nude and immunocompetent Sprague Dawley (SD) rats. At the end of both experiments, we could demonstrate that both rat types revealed comparably low levels of systemic IL-6 and IgM inflammation markers, as assessed via ELISA. Furthermore, the number of recovered cells was with no significant difference between both rat types. Conclusively, our results show that xenogeneic injection of human iMSC and NPC into immunoprivileged knee and IVD sites did not lead to an elevated inflammatory response in immunocompetent rats when compared to immunocompromised rats. Hence, immunocompetent rats represent suitable animals for xenotransplantation studies targeting immunoprivileged sites.
Keywords: PTOA; cell therapy; immune privilege; inflammation; intervertebral discs; joint; mesenchymal stem cells; nucleus pulposus cells; xenotransplantation.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Similar articles
-
Induced pluripotent stem cell-derived mesenchymal stem cells deliver exogenous miR-105-5p via small extracellular vesicles to rejuvenate senescent nucleus pulposus cells and attenuate intervertebral disc degeneration.Stem Cell Res Ther. 2021 May 13;12(1):286. doi: 10.1186/s13287-021-02362-1. Stem Cell Res Ther. 2021. PMID: 33985571 Free PMC article.
-
Mesenchymal stem cells deliver exogenous miR-21 via exosomes to inhibit nucleus pulposus cell apoptosis and reduce intervertebral disc degeneration.J Cell Mol Med. 2018 Jan;22(1):261-276. doi: 10.1111/jcmm.13316. Epub 2017 Aug 14. J Cell Mol Med. 2018. PMID: 28805297 Free PMC article.
-
Induced pluripotent stem cell-derived mesenchymal stem cells prolong hind limb survival in a rat vascularized composite allotransplantation model.Microsurgery. 2019 Nov;39(8):737-747. doi: 10.1002/micr.30507. Epub 2019 Aug 31. Microsurgery. 2019. PMID: 31471984
-
A Review: Methodologies to Promote the Differentiation of Mesenchymal Stem Cells for the Regeneration of Intervertebral Disc Cells Following Intervertebral Disc Degeneration.Cells. 2023 Aug 28;12(17):2161. doi: 10.3390/cells12172161. Cells. 2023. PMID: 37681893 Free PMC article. Review.
-
hiPSC-derived iMSCs: NextGen MSCs as an advanced therapeutically active cell resource for regenerative medicine.J Cell Mol Med. 2016 Aug;20(8):1571-88. doi: 10.1111/jcmm.12839. Epub 2016 Apr 21. J Cell Mol Med. 2016. PMID: 27097531 Free PMC article. Review.
Cited by
-
Advancements and Innovative Strategies in Induced Pluripotent Stem Cell-Derived Mesenchymal Stem Cell Therapy: A Comprehensive Review.Stem Cells Int. 2024 Sep 30;2024:4073485. doi: 10.1155/2024/4073485. eCollection 2024. Stem Cells Int. 2024. PMID: 39377039 Free PMC article. Review.
-
Therapeutic Potential of Umbilical Cord MSC-Derived Exosomes in a Severe Dry Eye Rat Model: Enhancing Corneal Protection and Modulating Inflammation.Biomedicines. 2025 May 11;13(5):1174. doi: 10.3390/biomedicines13051174. Biomedicines. 2025. PMID: 40427001 Free PMC article.
-
Collagen scaffold-seeded iTenocytes accelerate the healing and functional recovery of Achilles tendon defects in a rat model.Front Bioeng Biotechnol. 2024 Dec 6;12:1407729. doi: 10.3389/fbioe.2024.1407729. eCollection 2024. Front Bioeng Biotechnol. 2024. PMID: 39713100 Free PMC article.
References
Grants and funding
LinkOut - more resources
Full Text Sources

