The Potential of Deep Learning to Advance Clinical Applications of Computational Biomechanics
- PMID: 37760168
- PMCID: PMC10525821
- DOI: 10.3390/bioengineering10091066
The Potential of Deep Learning to Advance Clinical Applications of Computational Biomechanics
Abstract
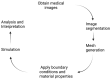
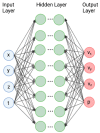
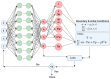
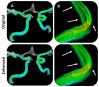
When combined with patient information provided by advanced imaging techniques, computational biomechanics can provide detailed patient-specific information about stresses and strains acting on tissues that can be useful in diagnosing and assessing treatments for diseases and injuries. This approach is most advanced in cardiovascular applications but can be applied to other tissues. The challenges for advancing computational biomechanics for real-time patient diagnostics and treatment include errors and missing information in the patient data, the large computational requirements for the numerical solutions to multiscale biomechanical equations, and the uncertainty over boundary conditions and constitutive relations. This review summarizes current efforts to use deep learning to address these challenges and integrate large data sets and computational methods to enable real-time clinical information. Examples are drawn from cardiovascular fluid mechanics, soft-tissue mechanics, and bone biomechanics. The application of deep-learning convolutional neural networks can reduce the time taken to complete image segmentation, and meshing and solution of finite element models, as well as improving the accuracy of inlet and outlet conditions. Such advances are likely to facilitate the adoption of these models to aid in the assessment of the severity of cardiovascular disease and the development of new surgical treatments.
Keywords: biomechanics; deep learning; finite element models; image segmentation; neural networks.
Conflict of interest statement
The author declares no conflict of interest.
Figures
Similar articles
-
Synergistic Integration of Deep Neural Networks and Finite Element Method with Applications of Nonlinear Large Deformation Biomechanics.Comput Methods Appl Mech Eng. 2023 Nov 1;416:116347. doi: 10.1016/j.cma.2023.116347. Epub 2023 Aug 22. Comput Methods Appl Mech Eng. 2023. PMID: 38370344 Free PMC article.
-
Robust automatic hexahedral cartilage meshing framework enables population-based computational studies of the knee.Front Bioeng Biotechnol. 2022 Dec 9;10:1059003. doi: 10.3389/fbioe.2022.1059003. eCollection 2022. Front Bioeng Biotechnol. 2022. PMID: 36568304 Free PMC article.
-
Semi-supervised learning for automatic segmentation of the knee from MRI with convolutional neural networks.Comput Methods Programs Biomed. 2020 Jun;189:105328. doi: 10.1016/j.cmpb.2020.105328. Epub 2020 Jan 11. Comput Methods Programs Biomed. 2020. PMID: 31958580
-
From Finite Element Meshes to Clouds of Points: A Review of Methods for Generation of Computational Biomechanics Models for Patient-Specific Applications.Ann Biomed Eng. 2016 Jan;44(1):3-15. doi: 10.1007/s10439-015-1469-2. Epub 2015 Sep 30. Ann Biomed Eng. 2016. PMID: 26424475 Review.
-
Machine Learning for Cardiovascular Biomechanics Modeling: Challenges and Beyond.Ann Biomed Eng. 2022 Jun;50(6):615-627. doi: 10.1007/s10439-022-02967-4. Epub 2022 Apr 20. Ann Biomed Eng. 2022. PMID: 35445297 Review.
Cited by
-
Computational Medicine: What Electrophysiologists Should Know to Stay Ahead of the Curve.Curr Cardiol Rep. 2024 Dec;26(12):1393-1403. doi: 10.1007/s11886-024-02136-0. Epub 2024 Sep 20. Curr Cardiol Rep. 2024. PMID: 39302590 Review.
-
[Numerical simulation in musculoskeletal biomechanics : Application perspectives and possibilities].Orthopadie (Heidelb). 2024 Jul;53(7):487-493. doi: 10.1007/s00132-024-04515-5. Epub 2024 Jun 3. Orthopadie (Heidelb). 2024. PMID: 38829399 German.
-
Helmet material design for mitigating traumatic axonal injuries through AI-driven constitutive law enhancement.Commun Eng. 2025 Feb 16;4(1):22. doi: 10.1038/s44172-025-00370-0. Commun Eng. 2025. PMID: 39956866 Free PMC article.
-
Advancing Meibography Assessment and Automated Meibomian Gland Detection Using Gray Value Profiles.Diagnostics (Basel). 2025 May 9;15(10):1199. doi: 10.3390/diagnostics15101199. Diagnostics (Basel). 2025. PMID: 40428192 Free PMC article.
References
-
- Humphrey J.D. Chapter 23—Enablers and drivers of vascular remodeling. In: Galis Z.S., editor. The Vasculome. Academic Press; Cambridge, MA, USA: 2022. pp. 277–285.
-
- Stefanati M., Villa C., Torrente Y., Rodriguez Matas J.F. A mathematical model of healthy and dystrophic skeletal muscle biomechanics. J. Mech. Phys. Solids. 2020;134:103747. doi: 10.1016/j.jmps.2019.103747. - DOI
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources

