Ultra-Low Intensity Post-Pulse Affects Cellular Responses Caused by Nanosecond Pulsed Electric Fields
- PMID: 37760171
- PMCID: PMC10525734
- DOI: 10.3390/bioengineering10091069
Ultra-Low Intensity Post-Pulse Affects Cellular Responses Caused by Nanosecond Pulsed Electric Fields
Abstract
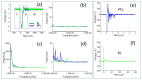
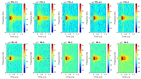
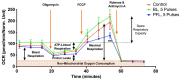
High-intensity nanosecond pulse electric fields (nsPEF) can preferentially induce various effects, most notably regulated cell death and tumor elimination. These effects have almost exclusively been shown to be associated with nsPEF waveforms defined by pulse duration, rise time, amplitude (electric field), and pulse number. Other factors, such as low-intensity post-pulse waveform, have been completely overlooked. In this study, we show that post-pulse waveforms can alter the cell responses produced by the primary pulse waveform and can even elicit unique cellular responses, despite the primary pulse waveform being nearly identical. We employed two commonly used pulse generator designs, namely the Blumlein line (BL) and the pulse forming line (PFL), both featuring nearly identical 100 ns pulse durations, to investigate various cellular effects. Although the primary pulse waveforms were nearly identical in electric field and frequency distribution, the post-pulses differed between the two designs. The BL's post-pulse was relatively long-lasting (~50 µs) and had an opposite polarity to the main pulse, whereas the PFL's post-pulse was much shorter (~2 µs) and had the same polarity as the main pulse. Both post-pulse amplitudes were less than 5% of the main pulse, but the different post-pulses caused distinctly different cellular responses. The thresholds for dissipation of the mitochondrial membrane potential, loss of viability, and increase in plasma membrane PI permeability all occurred at lower pulsing numbers for the PFL than the BL, while mitochondrial reactive oxygen species generation occurred at similar pulsing numbers for both pulser designs. The PFL decreased spare respiratory capacity (SRC), whereas the BL increased SRC. Only the PFL caused a biphasic effect on trans-plasma membrane electron transport (tPMET). These studies demonstrate, for the first time, that conditions resulting from low post-pulse intensity charging have a significant impact on cell responses and should be considered when comparing the results from similar pulse waveforms.
Keywords: charging current; intracellular effects; nanosecond pulse; post-pulse; spare respiratory capacity.
Conflict of interest statement
The authors declare no conflict of interest.
Figures






Similar articles
-
Modified Blumlein pulse-forming networks for bioelectrical applications.J Membr Biol. 2010 Jul;236(1):55-60. doi: 10.1007/s00232-010-9273-2. Epub 2010 Jul 7. J Membr Biol. 2010. PMID: 20607222
-
Nanosecond, high-intensity pulsed electric fields induce apoptosis in human cells.FASEB J. 2003 Aug;17(11):1493-5. doi: 10.1096/fj.02-0859fje. Epub 2003 Jun 17. FASEB J. 2003. PMID: 12824299
-
Effects of Nanosecond Pulsed Electric Field (nsPEF) on a Multicellular Spheroid Tumor Model: Influence of Pulse Duration, Pulse Repetition Rate, Absorbed Energy, and Temperature.Int J Mol Sci. 2023 Oct 8;24(19):14999. doi: 10.3390/ijms241914999. Int J Mol Sci. 2023. PMID: 37834447 Free PMC article.
-
[Research progress of nanosecond pulsed electric field applied to intracellular electromanipulation].Sheng Wu Yi Xue Gong Cheng Xue Za Zhi. 2008 Oct;25(5):1206-9. Sheng Wu Yi Xue Gong Cheng Xue Za Zhi. 2008. PMID: 19024477 Review. Chinese.
-
Bioelectric effects of intense ultrashort pulses.Crit Rev Biomed Eng. 2010;38(3):255-304. doi: 10.1615/critrevbiomedeng.v38.i3.20. Crit Rev Biomed Eng. 2010. PMID: 21133836 Review.
Cited by
-
Cardiomyocytes Permeabilization and Electrotransfection by Unipolar and Bipolar Asymmetric Electric Field Pulses.Bioelectricity. 2024 Jun 12;6(2):91-96. doi: 10.1089/bioe.2024.0001. eCollection 2024 Jun. Bioelectricity. 2024. PMID: 39119571 Free PMC article.
-
Special Issue "Electric, Magnetic, and Electromagnetic Fields in Biology and Medicine: From Mechanisms to Biomedical Applications: 2nd Edition".Bioengineering (Basel). 2025 Jul 7;12(7):739. doi: 10.3390/bioengineering12070739. Bioengineering (Basel). 2025. PMID: 40722431 Free PMC article.
References
-
- Beebe S.J., Joshi R., Schoenbach K.H., Xiao S. Ultrashort Electric Pulse Effects in Biology and Medicine. Springer; Singapore: 2021. - DOI
-
- Joshi R.P., Garner A.L., Sundararajan R. Review of Developments in Bioelectrics as an Application of Pulsed Power Technology. IEEE Trans. Plasma Sci. 2023;51:1682–1717. doi: 10.1109/TPS.2023.3281339. - DOI
-
- Pakhomov A.G., Kolb J.F., White J.A., Joshi R.P., Xiao S., Schoenbach K.H. Long-lasting plasma membrane permeabilization in mammalian cells by nanosecond pulsed electric field (nsPEF) Bioelectromagn. J. Bioelectromagn. Soc. Soc. Phys. Regul. Biol. Med. Eur. Bioelectromagn. Assoc. 2007;28:655–663. doi: 10.1002/bem.20354. - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

