Production of Astaxanthin by Animal Cells via Introduction of an Entire Astaxanthin Biosynthetic Pathway
- PMID: 37760175
- PMCID: PMC10525450
- DOI: 10.3390/bioengineering10091073
Production of Astaxanthin by Animal Cells via Introduction of an Entire Astaxanthin Biosynthetic Pathway
Abstract
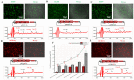
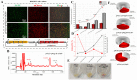
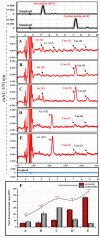
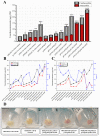
Astaxanthin is a fascinating molecule with powerful antioxidant activity, synthesized exclusively by specific microorganisms and higher plants. To expand astaxanthin production, numerous studies have employed metabolic engineering to introduce and optimize astaxanthin biosynthetic pathways in microorganisms and plant hosts. Here, we report the metabolic engineering of animal cells in vitro to biosynthesize astaxanthin. This was accomplished through a two-step study to introduce the entire astaxanthin pathway into human embryonic kidney cells (HEK293T). First, we introduced the astaxanthin biosynthesis sub-pathway (Ast subp) using several genes encoding β-carotene ketolase and β-carotene hydroxylase enzymes to synthesize astaxanthin directly from β-carotene. Next, we introduced a β-carotene biosynthesis sub-pathway (β-Car subp) with selected genes involved in Ast subp to synthesize astaxanthin from geranylgeranyl diphosphate (GGPP). As a result, we unprecedentedly enabled HEK293T cells to biosynthesize free astaxanthin from GGPP with a concentration of 41.86 µg/g dry weight (DW), which represented 66.19% of the total ketocarotenoids (63.24 µg/g DW). Through optimization steps using critical factors in the astaxanthin biosynthetic process, a remarkable 4.14-fold increase in total ketocarotenoids (262.10 µg/g DW) was achieved, with astaxanthin constituting over 88.82%. This pioneering study holds significant implications for transgenic animals, potentially revolutionizing the global demand for astaxanthin, particularly within the aquaculture sector.
Keywords: animal cells in vitro; astaxanthin; biosynthesis; enzymatic reactions; ketocarotenoids; metabolic engineering; multicistronic vectors; pathway.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



Similar articles
-
Engineering of a plasmid-free Escherichia coli strain for improved in vivo biosynthesis of astaxanthin.Microb Cell Fact. 2011 Apr 26;10:29. doi: 10.1186/1475-2859-10-29. Microb Cell Fact. 2011. PMID: 21521516 Free PMC article.
-
Reconstruction of the astaxanthin biosynthesis pathway in rice endosperm reveals a metabolic bottleneck at the level of endogenous β-carotene hydroxylase activity.Transgenic Res. 2017 Feb;26(1):13-23. doi: 10.1007/s11248-016-9977-x. Epub 2016 Aug 27. Transgenic Res. 2017. PMID: 27567632
-
Metabolic engineering of Saccharomyces cerevisiae for astaxanthin production and oxidative stress tolerance.Appl Environ Microbiol. 2009 Nov;75(22):7205-11. doi: 10.1128/AEM.01249-09. Epub 2009 Oct 2. Appl Environ Microbiol. 2009. PMID: 19801484 Free PMC article.
-
Metabolic engineering of ketocarotenoid biosynthesis in higher plants.Arch Biochem Biophys. 2009 Mar 15;483(2):182-90. doi: 10.1016/j.abb.2008.10.029. Epub 2008 Oct 29. Arch Biochem Biophys. 2009. PMID: 18992217 Review.
-
Canthaxanthin, a Red-Hot Carotenoid: Applications, Synthesis, and Biosynthetic Evolution.Plants (Basel). 2020 Aug 15;9(8):1039. doi: 10.3390/plants9081039. Plants (Basel). 2020. PMID: 32824217 Free PMC article. Review.
Cited by
-
Recent Advances in the Therapeutic Potential of Carotenoids in Preventing and Managing Metabolic Disorders.Plants (Basel). 2024 Jun 7;13(12):1584. doi: 10.3390/plants13121584. Plants (Basel). 2024. PMID: 38931016 Free PMC article. Review.
References
-
- Britton G., Liaaen-Jensen S., Pfander H., editors. Carotenoids: Handbook. Springer; Bronx, NY, USA: Birkhäuser; Basel, Switzerland: 2004.
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

