Cartilage Defect Treatment Using High-Density Autologous Chondrocyte Implantation (HD-ACI)
- PMID: 37760185
- PMCID: PMC10525711
- DOI: 10.3390/bioengineering10091083
Cartilage Defect Treatment Using High-Density Autologous Chondrocyte Implantation (HD-ACI)
Abstract
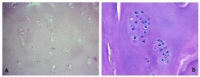
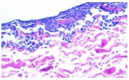
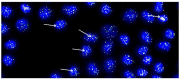
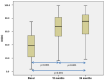
Hyaline cartilage's inability to self-repair can lead to osteoarthritis and joint replacement. Various treatments, including cell therapy, have been developed for cartilage damage. Autologous chondrocyte implantation (ACI) is considered the best option for focal chondral lesions. In this article, we aimed to create a narrative review that highlights the evolution and enhancement of our chondrocyte implantation technique: High-Density-ACI (HD-ACI) Membrane-assisted Autologous Chondrocyte Implantation (MACI) improved ACI using a collagen membrane as a carrier. However, low cell density in MACI resulted in softer regenerated tissue. HD-ACI was developed to improve MACI, implanting 5 million chondrocytes per cm2, providing higher cell density. In animal models, HD-ACI formed hyaline-like cartilage, while other treatments led to fibrocartilage. HD-ACI was further evaluated in patients with knee or ankle defects and expanded to treat hip lesions and bilateral defects. HD-ACI offers a potential solution for cartilage defects, improving outcomes in regenerative medicine and cell therapy. HD-ACI, with its higher cell density, shows promise for treating chondral defects and advancing cartilage repair in regenerative medicine and cell therapy.
Keywords: autologous chondrocyte implantation; cell therapy; cultured chondrocytes; high cell density; tissue engineering.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Similar articles
-
Autologous chondrocyte implantation versus matrix-induced autologous chondrocyte implantation for osteochondral defects of the knee: a prospective, randomised study.J Bone Joint Surg Br. 2005 May;87(5):640-5. doi: 10.1302/0301-620X.87B5.15905. J Bone Joint Surg Br. 2005. PMID: 15855365 Clinical Trial.
-
Prospective Long-term Follow-up of Autologous Chondrocyte Implantation With Periosteum Versus Matrix-Associated Autologous Chondrocyte Implantation: A Randomized Clinical Trial.Am J Sports Med. 2020 Jul;48(9):2230-2241. doi: 10.1177/0363546520928337. Am J Sports Med. 2020. PMID: 32667270 Clinical Trial.
-
Autologous chondrocyte implantation with collagen bioscaffold for the treatment of osteochondral defects in rabbits.Tissue Eng. 2005 Jul-Aug;11(7-8):1065-76. doi: 10.1089/ten.2005.11.1065. Tissue Eng. 2005. PMID: 16144442
-
Microfracture Versus Autologous Chondrocyte Implantation for Articular Cartilage Lesions in the Knee: A Systematic Review of 5-Year Outcomes.Am J Sports Med. 2018 Mar;46(4):995-999. doi: 10.1177/0363546517701912. Epub 2017 Apr 19. Am J Sports Med. 2018. PMID: 28423287
-
Is implantation of autologous chondrocytes superior to microfracture for articular-cartilage defects of the knee? A systematic review of 5-year follow-up data.Int J Surg. 2019 Aug;68:56-62. doi: 10.1016/j.ijsu.2019.06.007. Epub 2019 Jun 18. Int J Surg. 2019. PMID: 31220632
Cited by
-
Knee Arthroscopy in the Era of Precision Medicine: A Comprehensive Review of Tailored Approaches and Emerging Technologies.Cureus. 2024 Oct 6;16(10):e70932. doi: 10.7759/cureus.70932. eCollection 2024 Oct. Cureus. 2024. PMID: 39502973 Free PMC article. Review.
-
A review of advanced hydrogels for cartilage tissue engineering.Front Bioeng Biotechnol. 2024 Feb 8;12:1340893. doi: 10.3389/fbioe.2024.1340893. eCollection 2024. Front Bioeng Biotechnol. 2024. PMID: 38390359 Free PMC article. Review.
-
Latest Advances in Chondrocyte-Based Cartilage Repair.Biomedicines. 2024 Jun 19;12(6):1367. doi: 10.3390/biomedicines12061367. Biomedicines. 2024. PMID: 38927573 Free PMC article. Review.
-
Streamlined, single-step non-viral CRISPR-Cas9 knockout strategy enhances gene editing efficiency in primary human chondrocyte populations.Arthritis Res Ther. 2024 Mar 11;26(1):66. doi: 10.1186/s13075-024-03294-w. Arthritis Res Ther. 2024. PMID: 38468277 Free PMC article.
-
Influence of Cell Seeding Density and Material Stiffness on Chondrogenesis of Human Stem Cells Within Soft Hydrogels, Without the Use of Exogenous Growth Factors.Gels. 2025 Mar 18;11(3):213. doi: 10.3390/gels11030213. Gels. 2025. PMID: 40136918 Free PMC article.
References
Publication types
LinkOut - more resources
Full Text Sources

