Acclimation and Blood Sampling: Effects on Stress Markers in C57Bl/6J Mice
- PMID: 37760216
- PMCID: PMC10525122
- DOI: 10.3390/ani13182816
Acclimation and Blood Sampling: Effects on Stress Markers in C57Bl/6J Mice
Abstract
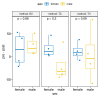
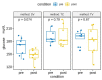
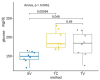
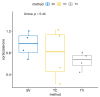
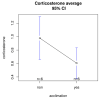
Blood sampling in rodents is common practice in scientific studies. Some of the refined methods widely used are the puncture of the saphenous vein or tail vein, or even tail docking. The handling needs of these different blood sampling methods are different and can directly affect stress, increasing the variability of the study. Moreover, there is less aversion and stress if the animal is accustomed to the environment, handling and technique. Therefore, our study aimed to assess the influence of these three blood sampling techniques (saphenous puncture, tail vein puncture and tail vein docking) and the use of previous acclimation on different indicators of animal stress, assessing blood glucose concentrations and faecal corticosterone metabolites (FCMs). Twenty-four young adult male and female C57Bl6/J mice were divided in three groups by sampling method: tail docking (TD), saphenous vein puncture (SV) and caudal vein puncture (CV) groups. All mice were studied with and without acclimation, which was performed during 9 consecutive days. The results showed that both males and females present very similar responses to the different handling and sampling methods without significant differences. Nevertheless, acclimation in all sampling methods decreased glucose and FCM levels significantly. The method that obtained the lowest glucose and FCM levels with significance was saphenous vein puncture. Therefore, we can say that it causes less stress when performing prior acclimation, even when this involves greater handling of the animal. Our results contribute to refinement within the 3R concept and could serve researchers to programme and select a good handling technique and a welfare-friendly blood sampling method for their experiments.
Keywords: 3R; acclimation; animal welfare; blood sampling; glucose refinement; mice; stress.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Similar articles
-
Comparison of sublingual, facial and retro-bulbar blood sampling in mice in relation to animal welfare and blood quality.J Pharmacol Toxicol Methods. 2020 May-Jun;103:106680. doi: 10.1016/j.vascn.2020.106680. Epub 2020 Feb 11. J Pharmacol Toxicol Methods. 2020. PMID: 32057916
-
Impact of three commonly used blood sampling techniques on the welfare of laboratory mice: Taking the animal's perspective.PLoS One. 2020 Sep 8;15(9):e0238895. doi: 10.1371/journal.pone.0238895. eCollection 2020. PLoS One. 2020. PMID: 32898190 Free PMC article.
-
A comparison of various methods of blood sampling in mice and rats: Effects on animal welfare.Lab Anim. 2018 Jun;52(3):253-264. doi: 10.1177/0023677217741332. Epub 2017 Nov 22. Lab Anim. 2018. PMID: 29165033
-
3R-Refinement principles: elevating rodent well-being and research quality.Lab Anim Res. 2024 Mar 29;40(1):11. doi: 10.1186/s42826-024-00198-3. Lab Anim Res. 2024. PMID: 38549171 Free PMC article. Review.
-
Best practices for non-lethal blood sampling of fish via the caudal vasculature.J Fish Biol. 2020 Jul;97(1):4-15. doi: 10.1111/jfb.14339. Epub 2020 May 29. J Fish Biol. 2020. PMID: 32243570 Review.
Cited by
-
Hyperactivity in male and female mice manifests differently following early, acute prenatal alcohol exposure and mild juvenile stress.Front Behav Neurosci. 2025 Mar 18;19:1501937. doi: 10.3389/fnbeh.2025.1501937. eCollection 2025. Front Behav Neurosci. 2025. PMID: 40170739 Free PMC article.
-
Chitosan Soft Matter Vesicles Loaded with Acetaminophen as Promising Systems for Modified Drug Release.Molecules. 2023 Dec 21;29(1):57. doi: 10.3390/molecules29010057. Molecules. 2023. PMID: 38202640 Free PMC article.
References
-
- National Centre for the Replacement Refinement & Reduction of Animal in Research (NC3Rs) Blood Sampling Mouse. NC3Rs; London, UK: 2013.
-
- Morton D.B., Abbot D., Barcley R., Close B.S., Ewbank R., Gask D., Heath M., Mattic S., Poole T., Seamer J., et al. Removal of blood from laboratory mammals and birds. First report of the BVA/FRAME/RSPCA/UFAW Joint Working Group on Refinement. Lab. Anim. 1993;27:1–22. - PubMed
-
- Dülsner A., Hack R., Krüger C., Pils M., Scherer K., Schmelting B., Schmidt M., Weinert H., Jourdan T. Recommendation for Blood Sampling in Laboratory Animals, Especially Small Laboratory Animals. Gesellschaft für Versuchstierkunde; Freiburg, Germany: 2017.
LinkOut - more resources
Full Text Sources

