Nociception in Chicken Embryos, Part II: Embryonal Development of Electroencephalic Neuronal Activity In Ovo as a Prerequisite for Nociception
- PMID: 37760239
- PMCID: PMC10525651
- DOI: 10.3390/ani13182839
Nociception in Chicken Embryos, Part II: Embryonal Development of Electroencephalic Neuronal Activity In Ovo as a Prerequisite for Nociception
Abstract
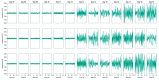
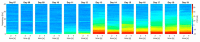
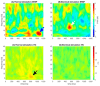
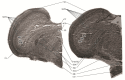
Chicken culling has been forbidden in Germany since 2022; male/female selection and male elimination must be brought to an embryonic status prior to the onset of nociception. The present study evaluated the ontogenetic point at which noxious stimuli could potentially be perceived/processed in the brain in ovo. EEG recordings from randomized hyperpallial brain sites were recorded in ovo and noxious stimuli were applied. Temporal and spectral analyses of the EEG were performed. The onset of physiological neuronal signals could be determined at developmental day 13. ERP/ERSP/ITC analysis did not reveal phase-locked nociceptive responses. Although no central nociceptive responses were documented, adequate EEG responses to noxious stimuli from other brain areas cannot be excluded. The extreme stress impact on the embryo during the recording may overwrite the perception of noniceptive stimuli. The results suggest developmental day 13 as the earliest embryonal stage being able to receive and process nociceptive stimuli.
Keywords: EEG; Gallus gallus domesticus; development; embryo; nociception; pain.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Krautwald-Junghanns M.E., Cramer K., Fischer B., Förster A., Galli R., Kremer F., Mapesa E.U., Meissner S., Preisinger R., Preusse G., et al. Current approaches to avoid the culling of day-old male chicks in the layer industry, with special reference to spectroscopic methods. Poult. Sci. 2017;97:749–757. doi: 10.3382/ps/pex389. - DOI - PubMed
-
- Gentle M.J., Hunter L.N. Physiological and behavioural responses associated with feather removal in Gallus gallus var domesticus. Res. Vet. Sci. 1990;50:95–101. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

