Is the Hippo Pathway Effector Yes-Associated Protein a Potential Key Player of Dairy Cattle Cystic Ovarian Disease Pathogenesis?
- PMID: 37760251
- PMCID: PMC10525513
- DOI: 10.3390/ani13182851
Is the Hippo Pathway Effector Yes-Associated Protein a Potential Key Player of Dairy Cattle Cystic Ovarian Disease Pathogenesis?
Abstract
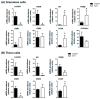
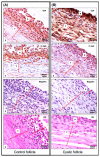
Cystic ovarian disease (COD) in dairy cattle is characterized by preovulatory follicles that become cysts, fail to ovulate and persist in the ovary; consequently, interfering with normal ovarian cyclicity. The intraovarian key players that orchestrate the alterations occurring in the preovulatory follicle and that culminate with cyst formation and persistence, however, remain uncertain. Interestingly, the Hippo pathway effector yes-associated protein (YAP) has been described in humans and mice as a key player of anovulatory cystic disorders. To start elucidating if YAP deregulation in ovarian follicle cells can be also involved in the pathogenesis of COD, we have generated a series of novel results using spontaneously occurring cystic follicles in cattle. We found that mRNA and protein levels of YAP are significantly higher in granulosa (GCs) and theca cells (TCs) isolated from cystic follicles (follicular structures of at least 20 mm in diameter) in comparison to respective cell types isolated from non-cystic large follicles (≥12 mm). In addition, immunohistochemistry and Western blot analyses used to determine YAP phosphorylation pattern suggest that YAP transcriptional activity is augmented is cystic GCs. These results were confirmed by a significant increase in the mRNA levels encoding for the classic YAP-TEAD transcriptional target genes CTGF, BIRC5 and ANKRD1 in GCs from follicle cysts in comparison to non-cystic large follicles. Taken together, these results provide considerable insight of a completely novel signaling pathway that seems to play an important role in ovarian cystic disease pathogenesis in dairy cattle.
Keywords: ANKRD1; COD; CTGF; CYR61; Hippo; TEAD; anovulation; cow; cyst; granulosa cells; ovary; theca cells.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



Similar articles
-
FSH Regulates YAP-TEAD Transcriptional Activity in Bovine Granulosa Cells to Allow the Future Dominant Follicle to Exert Its Augmented Estrogenic Capacity.Int J Mol Sci. 2022 Nov 16;23(22):14160. doi: 10.3390/ijms232214160. Int J Mol Sci. 2022. PMID: 36430640 Free PMC article.
-
The role of Hippo pathway signaling and A-kinase anchoring protein 13 in primordial follicle activation and inhibition.F S Sci. 2022 May;3(2):118-129. doi: 10.1016/j.xfss.2022.03.002. Epub 2022 Mar 30. F S Sci. 2022. PMID: 35560009 Free PMC article.
-
Estrogens receptors, nuclear coactivator 1 and ligand-dependent corepressor expression are altered early during induced ovarian follicular persistence in dairy cattle.Theriogenology. 2023 Oct 15;210:17-27. doi: 10.1016/j.theriogenology.2023.07.004. Epub 2023 Jul 13. Theriogenology. 2023. PMID: 37467695
-
A review on inflammation and angiogenesis as key mechanisms involved in the pathogenesis of bovine cystic ovarian disease.Theriogenology. 2022 Jul 1;186:70-85. doi: 10.1016/j.theriogenology.2022.04.002. Epub 2022 Apr 8. Theriogenology. 2022. PMID: 35430550 Review.
-
Contribution of key elements of nutritional metabolism to the development of cystic ovarian disease in dairy cattle.Theriogenology. 2023 Feb;197:209-223. doi: 10.1016/j.theriogenology.2022.12.003. Epub 2022 Dec 5. Theriogenology. 2023. PMID: 36525860 Review.
Cited by
-
Identifying the Role of YAP in the Development of Rumen Epithelium Using 3D Organoid.Stem Cells Int. 2025 Jul 11;2025:5105796. doi: 10.1155/sci/5105796. eCollection 2025. Stem Cells Int. 2025. PMID: 40689086 Free PMC article.
References
-
- Cattaneo L., Signorini M.L., Bertoli J., Bartolomé J.A., Gareis N.C., Díaz P.U., Bó G.A., Ortega H.H. Epidemiological description of cystic ovarian disease in argentine dairy herds: Risk factors and effects on the reproductive performance of lactating cows. Reprod. Domest. Anim.=Zuchthyg. 2014;49:1028–1033. doi: 10.1111/rda.12432. - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

