Zinc Oxide Nanoparticles (ZnO-NPs) Induce Cytotoxicity in the Zebrafish Olfactory Organs via Activating Oxidative Stress and Apoptosis at the Ultrastructure and Genetic Levels
- PMID: 37760268
- PMCID: PMC10525688
- DOI: 10.3390/ani13182867
Zinc Oxide Nanoparticles (ZnO-NPs) Induce Cytotoxicity in the Zebrafish Olfactory Organs via Activating Oxidative Stress and Apoptosis at the Ultrastructure and Genetic Levels
Abstract
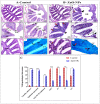
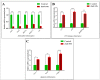
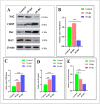
Nanotechnology has gained tremendous attention because of its crucial characteristics and wide biomedical applications. Although zinc oxide nanoparticles (ZnO-NPs) are involved in many industrial applications, researchers pay more attention to their toxic effects on living organisms. Since the olfactory epithelium is exposed to the external environment, it is considered the first organ affected by ZnO-NPs. Herein, we demonstrated the cytotoxic effect of ZnO-NPs on the olfactory organ of adult zebrafish after 60 days post-treatment. We opted for this period when fishes stop eating their diet from the aquarium, appear feeble, and cannot swim freely. Our study demonstrated that ZnO-NPs induced significant malformations of the olfactory rosettes at histological, ultrastructural, and genetic levels. At the ultrastructure level, the olfactory lamellae appeared collapsed, malformed, and twisted with signs of degeneration and loss of intercellular connections. In addition, ZnO-NPs harmed sensory receptor and ciliated cells, microvilli, rodlet, crypt, and Kappe cells, with hyper-activity of mucous secretion from goblet cells. At the genetic level, ZnO-NPs could activate the reactive oxygen species (ROS) synthesis expected by the down-regulation of mRNA expression for the antioxidant-related genes and up-regulation of DNA damage, cell growth arrest, and apoptosis. Interestingly, ZnO-NPs affected the odor sensation at 60 days post-treatment (60-dpt) more than at 30-dpt, severely damaging the olfactory epithelium and irreparably affecting the cellular repairing mechanisms. This induced a dramatically adverse effect on the cellular endoplasmic reticulum (ER), revealed by higher CHOP protein expression, that suppresses the antioxidant effect of Nrf2 and is followed by the induction of apoptosis via the up-regulation of Bax expression and down-regulation of Bcl-2 protein.
Keywords: apoptosis; olfactory epithelium; oxidative stress; zebrafish; zinc oxide nanoparticles (ZnO-NPs).
Conflict of interest statement
The authors declare no conflict of interest.
Figures




Similar articles
-
Oxidative stress and apotosis to zebrafish (Danio rerio) embryos exposed to perfluorooctane sulfonate (PFOS) and ZnO nanoparticles.Int J Occup Med Environ Health. 2017 Mar 30;30(2):213-229. doi: 10.13075/ijomeh.1896.00669. Epub 2017 Mar 17. Int J Occup Med Environ Health. 2017. PMID: 28366952
-
Zinc oxide nanoparticles exhibit cytotoxicity and genotoxicity through oxidative stress responses in human lung fibroblasts and Drosophila melanogaster.Int J Nanomedicine. 2017 Feb 28;12:1621-1637. doi: 10.2147/IJN.S124403. eCollection 2017. Int J Nanomedicine. 2017. PMID: 28280330 Free PMC article.
-
DBDPE and ZnO NPs synergistically induce neurotoxicity of SK-N-SH cells and activate mitochondrial apoptosis signaling pathway and Nrf2-mediated antioxidant pathway.J Hazard Mater. 2023 Jan 5;441:129872. doi: 10.1016/j.jhazmat.2022.129872. Epub 2022 Sep 3. J Hazard Mater. 2023. PMID: 36084461
-
Cutting-edge nanotechnology: unveiling the role of zinc oxide nanoparticles in combating deadly gastrointestinal tumors.Front Bioeng Biotechnol. 2025 Mar 20;13:1547757. doi: 10.3389/fbioe.2025.1547757. eCollection 2025. Front Bioeng Biotechnol. 2025. PMID: 40182988 Free PMC article. Review.
-
The Impact of Zinc Oxide Nanoparticles on Male (In)Fertility.Materials (Basel). 2020 Feb 13;13(4):849. doi: 10.3390/ma13040849. Materials (Basel). 2020. PMID: 32069903 Free PMC article. Review.
Cited by
-
Advances in antibacterial activity of zinc oxide nanoparticles against Staphylococcus aureus (Review).Biomed Rep. 2024 Aug 30;21(5):161. doi: 10.3892/br.2024.1849. eCollection 2024 Nov. Biomed Rep. 2024. PMID: 39268408 Free PMC article. Review.
-
Exploring Oxidative Stress Mechanisms of Nanoparticles Using Zebrafish (Danio rerio): Toxicological and Pharmaceutical Insights.Antioxidants (Basel). 2025 Apr 18;14(4):489. doi: 10.3390/antiox14040489. Antioxidants (Basel). 2025. PMID: 40298867 Free PMC article. Review.
-
Ultramicroscopic organization of the exterior olfactory organ in Anguilla vulgaris in relation to its spawning migration.Open Vet J. 2024 Jan;14(1):512-524. doi: 10.5455/OVJ.2024.v14.i1.46. Epub 2024 Jan 31. Open Vet J. 2024. PMID: 38633152 Free PMC article.
-
Ultrastructural and developmental anatomy of the peripheral olfactory organs of Dicentrarchus labrax inhabiting Egyptian Mediterranean water.Open Vet J. 2025 Feb;15(2):939-953. doi: 10.5455/OVJ.2025.v15.i2.43. Epub 2025 Feb 28. Open Vet J. 2025. PMID: 40201804 Free PMC article.
-
Ecological Risks of Zinc Oxide Nanoparticles for Early Life Stages of Obscure Puffer (Takifugu obscurus).Toxics. 2024 Jan 8;12(1):48. doi: 10.3390/toxics12010048. Toxics. 2024. PMID: 38251004 Free PMC article.
References
-
- Keller A.A., McFerran S., Lazareva A., Suh S. Global life cycle releases of engineered nanomaterials. J. Nanoparticle Res. 2013;15:1692. doi: 10.1007/s11051-013-1692-4. - DOI
-
- Piccinno F., Gottschalk F., Seeger S., Nowack B. Industrial production quantities and uses of ten engineered nanomaterials in Europe and the world. J. Nanoparticle Res. 2012;14:1109. doi: 10.1007/s11051-012-1109-9. - DOI
-
- Di Cerbo A., Mescola A., Iseppi R., Canton R., Rossi G., Stocchi R., Loschi A.R., Alessandrini A., Rea S., Sabia C. Antibacterial Effect of Aluminum Surfaces Untreated and Treated with a Special Anodizing Based on Titanium Oxide Approved for Food Contact. Biology. 2020;9:456. doi: 10.3390/biology9120456. - DOI - PMC - PubMed
-
- Di Cerbo A., Rosace G., Rea S., Stocchi R., Morales-Medina J.C., Canton R., Mescola A., Condo C., Loschi A.R., Sabia C. Time-Course Study of the Antibacterial Activity of an Amorphous SiO(x)C(y)H(z) Coating Certified for Food Contact. Antibiotics. 2021;10:901. doi: 10.3390/antibiotics10080901. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Research Materials

