Atypical Chronic Lymphocytic Leukemia-The Current Status
- PMID: 37760396
- PMCID: PMC10527541
- DOI: 10.3390/cancers15184427
Atypical Chronic Lymphocytic Leukemia-The Current Status
Abstract
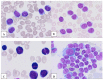
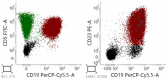
A diagnosis of typical chronic lymphocytic leukemia (CLL) requires the presence of ≥5000 clonal B-lymphocytes/μL, the coexistence of CD19, CD20, CD5, and CD23, the restriction of light chain immunoglobulin, and the lack of expression of antigens CD22 and CD79b. Atypical CLL (aCLL) can be distinguished from typical CLL morphologically and immunophenotypically. Morphologically atypical CLL cells have been defined mainly as large, atypical forms, prolymphocytes, or cleaved cells. However, current aCLL diagnostics rely more on immunophenotypic characteristics rather than atypical morphology. Immunophenotypically, atypical CLL differs from classic CLL in the lack of expression of one or fewer surface antigens, most commonly CD5 and CD23, and the patient does not meet the criteria for a diagnosis of any other B-cell lymphoid malignancy. Morphologically atypical CLL has more aggressive clinical behavior and worse prognosis than classic CLL. Patients with aCLL are more likely to display markers associated with poor prognosis, including trisomy 12, unmutated IGVH, and CD38 expression, compared with classic CLL. However, no standard or commonly accepted criteria exist for differentiating aCLL from classic CLL and the clinical significance of aCLL is still under debate. This review summarizes the current state of knowledge on the morphological, immunophenotypic, and genetic abnormalities of aCLL.
Keywords: CD23; CD5; CLL; CLL differential diagnosis; atypical CLL.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study, interpretation of the data, writing of the manuscript, or in the decision to publish the review.
Figures



References
-
- Hallek M., Cheson B.D., Catovsky D., Caligaris-Cappio F., Dighiero G., Dohner H., Hillmen P., Keating M., Montserrat E., Chiorazzi N., et al. iwCLL guidelines for diagnosis, indications for treatment, response assessment, and supportive management of CLL. Blood. 2018;131:2745–2760. doi: 10.1182/blood-2017-09-806398. - DOI - PubMed
-
- Eichhorst B., Robak T., Montserrat E., Ghia P., Niemann C.U., Kater A.P., Gregor M., Cymbalista F., Buske C., Hillmen P., et al. ESMO Guidelines Committee. Chronic lymphocytic leukaemia: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2021;32:23–33. doi: 10.1016/j.annonc.2020.09.019. - DOI - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Research Materials

