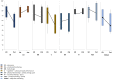
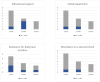
Quality of Life, Clinical, and Patient-Reported Outcomes after Pencil Beam Scanning Proton Therapy Delivered for Intracranial Grade WHO 1-2 Meningioma in Children and Adolescents
- PMID: 37760417
- PMCID: PMC10526222
- DOI: 10.3390/cancers15184447
Quality of Life, Clinical, and Patient-Reported Outcomes after Pencil Beam Scanning Proton Therapy Delivered for Intracranial Grade WHO 1-2 Meningioma in Children and Adolescents
Abstract
Purpose: The purpose of this study was to report the clinical and patient-reported outcomes of children and adolescents with intracranial meningioma treated with pencil beam scanning proton therapy (PBS-PT).
Material and methods: Out of a total cohort of 207 intracranial meningioma patients treated with PBS-PT between 1999 and 2022, 10 (4.8%) were children or adolescents aged < 18 years. Median age was 13.9 years (range, 3.2-17.2). Six (60%) children were treated as primary treatment (postoperative PT, n = 4; exclusive PT, n = 2) and four (40%) at the time of tumor recurrence. Acute and late toxicities were registered according to Common Terminology Criteria of Adverse Events (CTCAE). Quality of life (QoL) before PBS-PT was assessed using PEDQOL questionnaires. Educational, functional, and social aspects after PT were assessed through our in-house developed follow-up surveys. Median follow-up time was 71.1 months (range, 2.5-249.7), and median time to last questionnaire available was 37.6 months (range, 5.75-112.6).
Results: Five (50%) children developed local failure (LF) at a median time of 32.4 months (range, 17.7-55.4) after PBS-PT and four (80%) were considered in-field. One patient died of T-cell lymphoma 127.1 months after PBS-PT. Estimated 5-year local control (LC) and overall survival (OS) rates were 19.4% and 100.0%, respectively. Except for one patient who developed a cataract requiring surgery, no grade ≥3 late toxicities were reported. Before PT, patients rated their QoL lower than their parents in most domains. During the first year after PT, one child required educational support, one needed to attend to a special school, one had social problems and another three children required assistance for daily basic activities (DBA). Three years after PT, only one child required assistance for DBA.
Conclusions: The outcome of children with intracranial meningioma treated with PBS-PT is in line with other centers who have reported results of radiation therapy delivered to this particular patient group. This therapy provides acceptable functional status profiles with no high-grade adverse radiation-induced events.
Keywords: adolescents; children; intracranial meningioma; patient-reported outcomes; pencil beam scanning; proton therapy; quality of life; teenagers.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
Long Term Outcome and Quality of Life of Intracranial Meningioma Patients Treated with Pencil Beam Scanning Proton Therapy.Cancers (Basel). 2023 Jun 7;15(12):3099. doi: 10.3390/cancers15123099. Cancers (Basel). 2023. PMID: 37370709 Free PMC article.
-
Pencil Beam Scanning Proton Therapy for Adolescents and Young Adults with Head and Neck Sarcomas.Int J Part Ther. 2023 Oct 25;10(2):73-84. doi: 10.14338/IJPT-23-00010.1. eCollection 2023 Fall. Int J Part Ther. 2023. PMID: 38075481 Free PMC article.
-
Clinical outcomes and quality of life in children and adolescents with primary brain tumors treated with pencil beam scanning proton therapy.Pediatr Blood Cancer. 2020 Dec;67(12):e28465. doi: 10.1002/pbc.28465. Epub 2020 Sep 9. Pediatr Blood Cancer. 2020. PMID: 32902137
-
Proton Therapy for Intracranial Meningioma for the Treatment of Primary/Recurrent Disease Including Re-Irradiation.Front Oncol. 2020 Dec 14;10:558845. doi: 10.3389/fonc.2020.558845. eCollection 2020. Front Oncol. 2020. PMID: 33381447 Free PMC article. Review.
-
Proton Radiation Therapy for Head and Neck Cancers.Cureus. 2024 Oct 3;16(10):e70752. doi: 10.7759/cureus.70752. eCollection 2024 Oct. Cureus. 2024. PMID: 39493189 Free PMC article. Review.
Cited by
-
Overall survival and progression-free survival in pediatric meningiomas: a systematic review and individual patient-level meta-analysis.J Neurooncol. 2025 Apr;172(2):289-305. doi: 10.1007/s11060-024-04917-7. Epub 2025 Jan 9. J Neurooncol. 2025. PMID: 39779595 Free PMC article.
-
Non-cancer effects after proton beam therapy for pediatric tumors- a narrative review.Front Oncol. 2025 May 30;15:1554765. doi: 10.3389/fonc.2025.1554765. eCollection 2025. Front Oncol. 2025. PMID: 40519295 Free PMC article.
References
-
- Ostrom Q.T., De Blank P.M., Kruchko C., Petersen C.M., Liao P., Finlay J.L., Stearns D.S., Wolff J.E., Wolinsky Y., Letterio J.J., et al. Alex’s Lemonade Stand Foundation Infant and Childhood Primary Brain and Central Nervous System Tumors Diagnosed in the United States in 2007–2011. Neuro Oncol. 2014;16:x1–x35. doi: 10.1093/neuonc/nou223. - DOI - PMC - PubMed
-
- Ostrom Q.T., Gittleman H., De Blank P.M., Finlay J.L., Gurney J.G., McKean-Cowdin R., Stearns D.S., Wolff J.E., Liu M., Wolinsky Y., et al. American Brain Tumor Association Adolescent and Young Adult Primary Brain and Central Nervous System Tumors Diagnosed in the United States in 2008–2012. Neuro Oncol. 2015;18:i1–i50. doi: 10.1093/neuonc/nov297. - DOI - PMC - PubMed
-
- Kirches E., Sahm F., Korshunov A., Bluecher C., Waldt N., Kropf S., Schrimpf D., Sievers P., Stichel D., Schüller U., et al. Molecular Profiling of Pediatric Meningiomas Shows Tumor Characteristics Distinct from Adult Meningiomas. Acta Neuropathol. 2021;142:873–886. doi: 10.1007/s00401-021-02351-x. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources