Radiolabeled NGR-Based Heterodimers for Angiogenesis Imaging: A Review of Preclinical Studies
- PMID: 37760428
- PMCID: PMC10526435
- DOI: 10.3390/cancers15184459
Radiolabeled NGR-Based Heterodimers for Angiogenesis Imaging: A Review of Preclinical Studies
Abstract
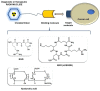
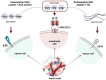
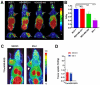
Since angiogenesis/neoangiogenesis has a major role in tumor development, progression and metastatic spread, the establishment of angiogenesis-targeting imaging and therapeutic vectors is of utmost significance. Aminopeptidase N (APN/CD13) is a pivotal biomarker of angiogenic processes abundantly expressed on the cell surface of active vascular endothelial and various neoplastic cells, constituting a valuable target for cancer diagnostics and therapy. Since the asparagine-glycine-arginine (NGR) sequence has been shown to colocalize with APN/CD13, the research interest in NGR-peptide-mediated vascular targeting is steadily growing. Earlier preclinical experiments have already demonstrated the imaging and therapeutic feasibility of NGR-based probes labeled with different positron emission tomography (PET) and single-photon emission computed tomography (SPECT) radionuclides, including Gallium-68 (68Ga), Copper-64 (64Cu), Technetium-99m (99mTc), Lutetium-177 (177Lu), Rhenium-188 (188Re) or Bismuth-213 (213Bi). To improve the tumor binding affinity and the retention time of single-receptor targeting peptides, NGR motifs containing heterodimers have been introduced to identify multi-receptor overexpressing malignancies. Preclinical studies with various tumor-bearing experimental animals provide useful tools for the investigation of the in vivo imaging behavior of NGR-based heterobivalent ligands. Herein, we review the reported preclinical achievements on NGR heterodimers that could be highly relevant for the development of further target-specific multivalent compounds in diagnostic and therapeutic settings.
Keywords: Aminopeptidase N (APN/CD13); angiogenesis; asparagine–glycine–arginine (NGR); heterodimer; preclinical; radiolabeled.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
NGR-Based Radiopharmaceuticals for Angiogenesis Imaging: A Preclinical Review.Int J Mol Sci. 2023 Aug 11;24(16):12675. doi: 10.3390/ijms241612675. Int J Mol Sci. 2023. PMID: 37628856 Free PMC article. Review.
-
In vivo assessment of aminopeptidase N (APN/CD13) specificity of different 68Ga-labelled NGR derivatives using PET/MRI imaging.Int J Pharm. 2020 Nov 15;589:119881. doi: 10.1016/j.ijpharm.2020.119881. Epub 2020 Sep 16. Int J Pharm. 2020. PMID: 32946975
-
In vivo imaging of Aminopeptidase N (CD13) receptors in experimental renal tumors using the novel radiotracer (68)Ga-NOTA-c(NGR).Eur J Pharm Sci. 2015 Mar 10;69:61-71. doi: 10.1016/j.ejps.2015.01.002. Epub 2015 Jan 13. Eur J Pharm Sci. 2015. PMID: 25592229
-
Synthesis and evaluation of 68Ga-labeled dimeric cNGR peptide for PET imaging of CD13 expression with ovarian cancer xenograft.J Cancer. 2021 Jan 1;12(1):244-252. doi: 10.7150/jca.49628. eCollection 2021. J Cancer. 2021. PMID: 33391421 Free PMC article.
-
Scandium-44: Diagnostic Feasibility in Tumor-Related Angiogenesis.Int J Mol Sci. 2023 Apr 17;24(8):7400. doi: 10.3390/ijms24087400. Int J Mol Sci. 2023. PMID: 37108559 Free PMC article. Review.
Cited by
-
Peptide-Mediated Nanocarriers for Targeted Drug Delivery: Developments and Strategies.Pharmaceutics. 2024 Feb 6;16(2):240. doi: 10.3390/pharmaceutics16020240. Pharmaceutics. 2024. PMID: 38399294 Free PMC article. Review.
-
NGR-modified nanovesicles target ALKBH5 to inhibit ovarian cancer growth and metastasis.Theranostics. 2025 Jun 9;15(14):6702-6718. doi: 10.7150/thno.107766. eCollection 2025. Theranostics. 2025. PMID: 40585991 Free PMC article.
-
NGR-modified curcumin nanovesicles reverse immunotherapy resistance in triple-negative breast cancer via TLR9 and mTOR pathway modulation.Cell Biol Toxicol. 2025 Jul 1;41(1):109. doi: 10.1007/s10565-025-10055-1. Cell Biol Toxicol. 2025. PMID: 40590998 Free PMC article.
-
NGR-Modified CAF-Derived exos Targeting Tumor Vasculature to Induce Ferroptosis and Overcome Chemoresistance in Osteosarcoma.Adv Sci (Weinh). 2025 Mar;12(12):e2410918. doi: 10.1002/advs.202410918. Epub 2025 Jan 31. Adv Sci (Weinh). 2025. PMID: 39889249 Free PMC article.
References
Publication types
LinkOut - more resources
Full Text Sources
Miscellaneous

