Outcome of Completion Surgery after Endoscopic Submucosal Dissection in Early-Stage Colorectal Cancer Patients
- PMID: 37760458
- PMCID: PMC10526268
- DOI: 10.3390/cancers15184490
Outcome of Completion Surgery after Endoscopic Submucosal Dissection in Early-Stage Colorectal Cancer Patients
Abstract
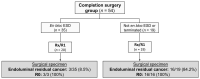
T1 colorectal cancers (T1CRC) are increasingly being treated by endoscopic submucosal dissection (ESD). After ESD of a T1CRC, completion surgery is indicated in a subgroup of patients. Currently, the influence of ESD on surgical morbidity and mortality is unknown. The aim of this study was to compare 90-day morbidity and mortality of completion surgery after ESD to primary surgery. The completion surgery group consisted of suspected T1CRC patients from a multicenter prospective ESD database (2014-2020). The primary surgery group consisted of pT1CRC patients from a nationwide surgical registry (2017-2019). Patients with rectal or sigmoidal cancers were selected. Patients receiving neoadjuvant therapy were excluded. Propensity score adjustment was used to correct for confounders. In total, 411 patients were included: 54 in the completion surgery group (39 pT1, 15 pT2) and 357 in the primary surgery group with pT1CRC. Adverse event rate was 24.1% after completion surgery and 21.3% after primary surgery. After completion surgery 90-day mortality did not occur, though one patient died in the primary surgery group. After propensity score adjustment, lymph node yield did not differ significantly between the groups. Among other morbidity-related outcomes, stoma rate (OR 1.298 95%-CI 0.587-2.872, p = 0.519) and adverse event rate (OR 1.162; 95%-CI 0.570-2.370, p = 0.679) also did not differ significantly. A subgroup analysis was performed in patients undergoing rectal surgery. In this subgroup (37 completion and 136 primary surgery), these morbidity outcomes also did not differ significantly. In conclusion, this study suggests that ESD does not compromise morbidity or 90-day mortality of completion surgery.
Keywords: T1CRC; colorectal cancer; completion surgery; endoscopic submucosal dissection; morbidity; nationwide database; total mesorectal excision.
Conflict of interest statement
Leon M. G. Moons is a consultant for Boston Scientific. This disclosure does not directly relate to the content of this work. All other authors declare that they have no competing interest.
Figures
References
-
- Dang H., Dekkers N., le Cessie S., van Hooft J.E., van Leerdam M.E., Oldenburg P.P., Flothuis L., Schoones J.W., Langers A.M.J., Hardwick J.C.H., et al. Risk and Time Pattern of Recurrences After Local Endoscopic Resection of T1 Colorectal Cancer: A Meta-analysis. Clin. Gastroenterol. Hepatol. 2020;20:e298–e314. doi: 10.1016/j.cgh.2020.11.032. - DOI - PubMed
-
- Dekkers N., Dang H., van der Kraan J., le Cessie S., Oldenburg P.P., Schoones J.W., Langers A.M.J., van Leerdam M.E., van Hooft J.E., Backes Y., et al. Risk of recurrence after local resection of T1 rectal cancer: A meta-analysis with meta-regression. Surg. Endosc. 2022;36:9156–9168. doi: 10.1007/s00464-022-09396-3. - DOI - PMC - PubMed
-
- Wang A.Y., Hwang J.H., Bhatt A., Draganov P.V. AGA Clinical Practice Update on Surveillance After Pathologically Curative Endoscopic Submucosal Dissection of Early Gastrointestinal Neoplasia in the United States: Commentary. Gastroenterology. 2021;161:2030–2040.e1. doi: 10.1053/j.gastro.2021.08.058. - DOI - PubMed
-
- Hashiguchi Y., Muro K., Saito Y., Ito Y., Ajioka Y., Hamaguchi T., Hasegawa K., Hotta K., Ishida H., Ishiguro M., et al. Japanese Society for Cancer of the Colon and Rectum (JSCCR) guidelines 2019 for the treatment of colorectal cancer. Int. J. Clin. Oncol. 2020;25:1–42. doi: 10.1007/s10147-019-01485-z. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous