Feasibility and Activity of Megestrol Acetate in Addition to Etoposide, Doxorubicin, Cisplatin, and Mitotane as First-Line Therapy in Patients with Metastatic/Unresectable Adrenocortical Carcinoma with Low Performance Status
- PMID: 37760461
- PMCID: PMC10527072
- DOI: 10.3390/cancers15184491
Feasibility and Activity of Megestrol Acetate in Addition to Etoposide, Doxorubicin, Cisplatin, and Mitotane as First-Line Therapy in Patients with Metastatic/Unresectable Adrenocortical Carcinoma with Low Performance Status
Abstract
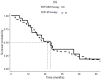
(1) Background: The standard first-line therapy for advanced adrenocortical carcinoma (ACC) is represented by EDP-M (etoposide, doxorubicin, cisplatin + mitotane). Progestins have shown cytotoxic activity both in vitro and in vivo on ACC; better EDP-M tolerability and efficacy have been hypnotized due to the association with progestins. (2) Methods: The feasibility and tolerability of EDP-M combined with oral megestrol acetate (EDP-MM) were tested in 24 patients (pts) affected by metastatic ACC with a low performance status (PS); the case group was compared with a 48 pts control group according to the propensity score. The secondary objectives were clinical benefit rate (CBR), progression-free survival (PFS), and overall survival (OS). (3) Results: Thirteen pts (54.2%) in the EDP-MM population experienced progestin-related toxicities; in particular, five pts experienced vaginal bleeding (20.8%); four pts experienced weight gain (16.7%); and thromboembolic events, worsening of hypertension, skin rashes, and hyperglycemia were registered in one patient each (4.2%). This led to the discontinuation of megestrol acetate in four pts (16.7%). EDP-M-related toxicities were similar in both groups. No differences in PFS and OS curves were observed; the CBR was 75.0% and 60.4%, respectively. (4) Conclusions: The association of EDP-M + megestrol acetate in ACC pts with a low PS is feasible and well tolerated; its efficacy appeared to be non-inferior to EDP-M administered to pts with a good PS.
Keywords: adrenocortical carcinoma; chemotherapy; megestrol acetate.
Conflict of interest statement
A.B. received research funds for ACC studies from Janssen, Sanofi, Novartis. All the other authors declare no conflict of interest.
Figures

Similar articles
-
Clinical management and outcomes associated with etoposide, doxorubicin, and cisplatin plus mitotane treatment in metastatic adrenocortical carcinoma: a single institute experience.Int J Clin Oncol. 2021 Dec;26(12):2275-2281. doi: 10.1007/s10147-021-02021-8. Epub 2021 Sep 1. Int J Clin Oncol. 2021. PMID: 34468885 Clinical Trial.
-
Preclinical progress and first translational steps for a liposomal chemotherapy protocol against adrenocortical carcinoma.Endocr Relat Cancer. 2016 Oct;23(10):825-37. doi: 10.1530/ERC-16-0249. Epub 2016 Aug 22. Endocr Relat Cancer. 2016. PMID: 27550961
-
Mitotane associated with etoposide, doxorubicin, and cisplatin in the treatment of advanced adrenocortical carcinoma. Italian Group for the Study of Adrenal Cancer.Cancer. 1998 Nov 15;83(10):2194-200. Cancer. 1998. PMID: 9827725 Clinical Trial.
-
Supportive therapies in patients with advanced adrenocortical carcinoma submitted to standard EDP-M regimen.Endocrine. 2022 Sep;77(3):438-443. doi: 10.1007/s12020-022-03075-y. Epub 2022 May 14. Endocrine. 2022. PMID: 35567656 Free PMC article. Review.
-
Advances in adrenocortical carcinoma pharmacotherapy: what is the current state of the art?Expert Opin Pharmacother. 2022 Aug;23(12):1413-1424. doi: 10.1080/14656566.2022.2106128. Epub 2022 Aug 3. Expert Opin Pharmacother. 2022. PMID: 35876101 Review.
References
-
- Fassnacht M., Assie G., Baudin E., Eisenhofer G., de la Fouchardiere C., Haak H.R., de Krijger R., Porpiglia F., Terzolo M., Berruti A. Adrenocortical Carcinomas and Malignant Phaeochromocytomas: ESMO–EURACAN Clinical Practice Guidelines for Diagnosis, Treatment and Follow-Up. Ann. Oncol. 2020;31:1476–1490. doi: 10.1016/j.annonc.2020.08.2099. - DOI - PubMed
-
- Berruti A., Terzolo M., Sperone P., Pia A., Della Casa S., Gross D.J., Carnaghi C., Casali P., Porpiglia F., Mantero F., et al. Etoposide, Doxorubicin and Cisplatin plus Mitotane in the Treatment of Advanced Adrenocortical Carcinoma: A Large Prospective Phase II Trial. Endocr. Relat. Cancer. 2005;12:657–666. doi: 10.1677/erc.1.01025. - DOI - PubMed
-
- Elhassan Y.S., Altieri B., Berhane S., Cosentini D., Calabrese A., Haissaguerre M., Kastelan D., Fragoso M.C.B.V., Bertherat J., Al Ghuzlan A., et al. S-GRAS Score for Prognostic Classification of Adrenocortical Carcinoma: An International, Multicenter ENSAT Study. Eur. J. Endocrinol. 2022;186:25–36. doi: 10.1530/EJE-21-0510. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

