Artificial Intelligence in Digital Pathology for Bladder Cancer: Hype or Hope? A Systematic Review
- PMID: 37760487
- PMCID: PMC10526515
- DOI: 10.3390/cancers15184518
Artificial Intelligence in Digital Pathology for Bladder Cancer: Hype or Hope? A Systematic Review
Abstract
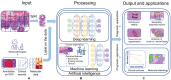
Bladder cancer (BC) diagnosis and prediction of prognosis are hindered by subjective pathological evaluation, which may cause misdiagnosis and under-/over-treatment. Computational pathology (CPATH) can identify clinical outcome predictors, offering an objective approach to improve prognosis. However, a systematic review of CPATH in BC literature is lacking. Therefore, we present a comprehensive overview of studies that used CPATH in BC, analyzing 33 out of 2285 identified studies. Most studies analyzed regions of interest to distinguish normal versus tumor tissue and identify tumor grade/stage and tissue types (e.g., urothelium, stroma, and muscle). The cell's nuclear area, shape irregularity, and roundness were the most promising markers to predict recurrence and survival based on selected regions of interest, with >80% accuracy. CPATH identified molecular subtypes by detecting features, e.g., papillary structures, hyperchromatic, and pleomorphic nuclei. Combining clinicopathological and image-derived features improved recurrence and survival prediction. However, due to the lack of outcome interpretability and independent test datasets, robustness and clinical applicability could not be ensured. The current literature demonstrates that CPATH holds the potential to improve BC diagnosis and prediction of prognosis. However, more robust, interpretable, accurate models and larger datasets-representative of clinical scenarios-are needed to address artificial intelligence's reliability, robustness, and black box challenge.
Keywords: artificial intelligence; bladder cancer; computational pathology; computer-aided diagnosis; digital pathology; histopathology; image analysis.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Figures
Similar articles
-
Computational pathology: A survey review and the way forward.J Pathol Inform. 2024 Jan 14;15:100357. doi: 10.1016/j.jpi.2023.100357. eCollection 2024 Dec. J Pathol Inform. 2024. PMID: 38420608 Free PMC article. Review.
-
Application of Artificial Intelligence in Pathology: Trends and Challenges.Diagnostics (Basel). 2022 Nov 15;12(11):2794. doi: 10.3390/diagnostics12112794. Diagnostics (Basel). 2022. PMID: 36428854 Free PMC article. Review.
-
Computational pathology for breast cancer: Where do we stand for prognostic applications?Breast. 2025 Jun;81:104464. doi: 10.1016/j.breast.2025.104464. Epub 2025 Mar 26. Breast. 2025. PMID: 40179582 Free PMC article. Review.
-
Towards a general-purpose foundation model for computational pathology.Nat Med. 2024 Mar;30(3):850-862. doi: 10.1038/s41591-024-02857-3. Epub 2024 Mar 19. Nat Med. 2024. PMID: 38504018 Free PMC article.
-
Pathologists' first opinions on barriers and facilitators of computational pathology adoption in oncological pathology: an international study.Oncogene. 2023 Sep;42(38):2816-2827. doi: 10.1038/s41388-023-02797-1. Epub 2023 Aug 16. Oncogene. 2023. PMID: 37587332 Free PMC article.
Cited by
-
Equipping computational pathology systems with artifact processing pipelines: a showcase for computation and performance trade-offs.BMC Med Inform Decis Mak. 2024 Oct 7;24(1):288. doi: 10.1186/s12911-024-02676-z. BMC Med Inform Decis Mak. 2024. PMID: 39375719 Free PMC article.
-
NMGrad: Advancing Histopathological Bladder Cancer Grading with Weakly Supervised Deep Learning.Bioengineering (Basel). 2024 Sep 11;11(9):909. doi: 10.3390/bioengineering11090909. Bioengineering (Basel). 2024. PMID: 39329651 Free PMC article.
-
Intervention of machine learning in bladder cancer research using multi-omics datasets: systematic review on biomarker identification.Discov Oncol. 2025 Jun 5;16(1):1010. doi: 10.1007/s12672-025-02734-6. Discov Oncol. 2025. PMID: 40471489 Free PMC article. Review.
-
ELW-CNN: An extremely lightweight convolutional neural network for enhancing interoperability in colon and lung cancer identification using explainable AI.Healthc Technol Lett. 2025 Jan 22;12(1):e12122. doi: 10.1049/htl2.12122. eCollection 2025 Jan-Dec. Healthc Technol Lett. 2025. PMID: 39845172 Free PMC article.
-
Artificial intelligence for diagnosing bladder pathophysiology: An updated review and future prospects.Bladder (San Franc). 2025 Apr 10;12(2):e21200042. doi: 10.14440/bladder.2024.0054. eCollection 2025. Bladder (San Franc). 2025. PMID: 40747464 Free PMC article. Review.
References
-
- Woerl A.-C., Eckstein M., Geiger J., Wagner D.C., Daher T., Stenzel P., Fernandez A., Hartmann A., Wand M., Roth W., et al. Deep Learning Predicts Molecular Subtype of Muscle-invasive Bladder Cancer from Conventional Histopathological Slides. Eur. Urol. 2020;78:256–264. doi: 10.1016/j.eururo.2020.04.023. - DOI - PubMed
-
- Zhang Z., Chen P., McGough M., Xing F., Wang C., Bui M., Xie Y., Sapkota M., Cui L., Dhillon J., et al. Pathologist-level interpretable whole-slide cancer diagnosis with deep learning. Nat. Mach. Intell. 2019;1:236–245. doi: 10.1038/s42256-019-0052-1. - DOI
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources

