Neo-Adjuvant Treatment in Primary Resectable Pancreatic Cancer: A Systematic Review and PRISMA-Compliant Updated Metanalysis of Oncological Outcomes
- PMID: 37760596
- PMCID: PMC10526896
- DOI: 10.3390/cancers15184627
Neo-Adjuvant Treatment in Primary Resectable Pancreatic Cancer: A Systematic Review and PRISMA-Compliant Updated Metanalysis of Oncological Outcomes
Abstract
Background: Despite advances in treatment, the prognosis of resectable pancreatic adenocarcinoma remains poor. Neoadjuvant therapy (NAT) has gained great interest in hopes of improving survival. However, the results of available studies based on different treatment approaches, such as chemotherapy and chemoradiotherapy, showed contrasting results. The aim of this systematic review and meta-analysis is to clarify the benefit of NAT compared to upfront surgery (US) in primarily resectable pancreatic adenocarcinoma.
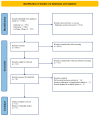
Methods: A PRISMA literature review identified 139 studies, of which 15 were finally included in the systematic review and meta-analysis. All data from eligible articles was summarized in a systematic summary and then used for the meta-analysis. Specifically, we used HR for OS and DFS and risk estimates (odds ratios) for the R0 resection rate and the N+ rate. The risk of bias was correctly assessed according to the nature of the studies included.
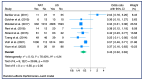
Results: From the pooled HRs, OS for NAT patients was better, with an HR for death of 0.80 (95% CI: 0.72-0.90) at a significance level of less than 1%. In the sub-group analysis, no difference was found between patients treated with chemoradiotherapy or chemotherapy exclusively. The meta-analysis of seven studies that reported DFS for NAT resulted in a pooled HR for progression of 0.66 (95% CI: 0.56-0.79) with a significance level of less than 1%. A significantly lower risk of positive lymph nodes (OR: 0.45; 95% CI: 0.32-0.63) and an improved R0 resection rate (OR: 1.70; 95% CI: 1.23-2.36) were also found in patients treated with NAT, despite high heterogeneity.
Conclusions: NAT is associated with improved survival for patients with resectable pancreatic adenocarcinoma; however, the optimal treatment strategy has yet to be defined, and further studies are required.
Keywords: neo-adjuvant treatment; neoadjuvant chemoradiotherapy; neoadjuvant chemotherapy; pancreatic adenocarcinoma; resectable pancreatic cancer.
Conflict of interest statement
The authors declare the absence of any commercial or financial relationship that could be construed as a potential conflicts of interest.
Figures





References
-
- Tempero M.A., Malafa M.P., Al-Hawary M., Behrman S.W., Benson A.B., Cardin D.B., Chiorean E.G., Chung V., Czito B., Del Chiaro M., et al. Pancreatic Adenocarcinoma, Version 2.2021, NCCN Clinical Practice Guidelines in Oncology. J. Natl. Compr. Canc Netw. 2021;19:439–457. doi: 10.6004/jnccn.2021.0017. - DOI - PubMed
-
- Al-Hawary M.M., Francis I.R., Chari S.T., Fishman E.K., Hough D.M., Lu D.S., Macari M., Megibow A.J., Miller F.H., Mortele K.J., et al. Pancreatic ductal adenocarcinoma radiology reporting template: Consensus statement of the society of abdominal radiology and the american pancreatic association. Gastroenterology. 2014;146:291–304.e291. doi: 10.1053/j.gastro.2013.11.004. - DOI - PubMed
Publication types
LinkOut - more resources
Full Text Sources

