Natural Products and Small Molecules Targeting Cellular Ceramide Metabolism to Enhance Apoptosis in Cancer Cells
- PMID: 37760612
- PMCID: PMC10527029
- DOI: 10.3390/cancers15184645
Natural Products and Small Molecules Targeting Cellular Ceramide Metabolism to Enhance Apoptosis in Cancer Cells
Abstract
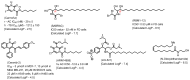
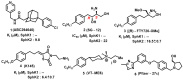
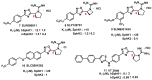
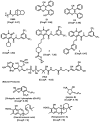
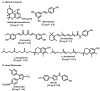
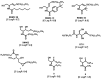
Molecular targeting strategies have been used for years in order to control cancer progression and are often based on targeting various enzymes involved in metabolic pathways. Keeping this in mind, it is essential to determine the role of each enzyme in a particular metabolic pathway. In this review, we provide in-depth information on various enzymes such as ceramidase, sphingosine kinase, sphingomyelin synthase, dihydroceramide desaturase, and ceramide synthase which are associated with various types of cancers. We also discuss the physicochemical properties of well-studied inhibitors with natural product origins and their related structures in terms of these enzymes. Targeting ceramide metabolism exhibited promising mono- and combination therapies at preclinical stages in preventing cancer progression and cemented the significance of sphingolipid metabolism in cancer treatments. Targeting ceramide-metabolizing enzymes will help medicinal chemists design potent and selective small molecules for treating cancer progression at various levels.
Keywords: anticancer therapies; ceramide; ceramide synthase; natural products and related small molecules; sphingolipids.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Similar articles
-
Novel agents targeting bioactive sphingolipids for the treatment of cancer.Curr Med Chem. 2013;20(1):108-22. Curr Med Chem. 2013. PMID: 23244584 Review.
-
Functions of neutral ceramidase in the Golgi apparatus.J Lipid Res. 2018 Nov;59(11):2116-2125. doi: 10.1194/jlr.M088187. Epub 2018 Aug 28. J Lipid Res. 2018. PMID: 30154232 Free PMC article.
-
The unfolding role of ceramide in coordinating retinoid-based cancer therapy.Biochem J. 2021 Oct 15;478(19):3621-3642. doi: 10.1042/BCJ20210368. Biochem J. 2021. PMID: 34648006 Review.
-
Ceramide synthase inhibition by fumonisin B1 treatment activates sphingolipid-metabolizing systems in mouse liver.Toxicol Sci. 2006 Dec;94(2):388-97. doi: 10.1093/toxsci/kfl102. Epub 2006 Sep 7. Toxicol Sci. 2006. PMID: 16960033
-
Increased killing of SCCVII squamous cell carcinoma cells after the combination of Pc 4 photodynamic therapy and dasatinib is associated with enhanced caspase-3 activity and ceramide synthase 1 upregulation.Int J Oncol. 2013 Dec;43(6):2064-72. doi: 10.3892/ijo.2013.2132. Epub 2013 Oct 9. Int J Oncol. 2013. PMID: 24126464 Free PMC article.
Cited by
-
Don't Be Surprised When These Surprise You: Some Infrequently Studied Sphingoid Bases, Metabolites, and Factors That Should Be Kept in Mind During Sphingolipidomic Studies.Int J Mol Sci. 2025 Jan 14;26(2):650. doi: 10.3390/ijms26020650. Int J Mol Sci. 2025. PMID: 39859363 Free PMC article. Review.
-
Hepatotoxicity assessment of innovative nutritional supplements based on olive-oil formulations enriched with natural antioxidants.Front Nutr. 2024 May 15;11:1388492. doi: 10.3389/fnut.2024.1388492. eCollection 2024. Front Nutr. 2024. PMID: 38812942 Free PMC article.
-
Mysterious sphingolipids: metabolic interrelationships at the center of pathophysiology.Front Physiol. 2024 Jan 3;14:1229108. doi: 10.3389/fphys.2023.1229108. eCollection 2023. Front Physiol. 2024. PMID: 38235387 Free PMC article. Review.
-
Bioactive lipid signaling and lipidomics in macrophage polarization: Impact on inflammation and immune regulation.Front Immunol. 2025 Feb 14;16:1550500. doi: 10.3389/fimmu.2025.1550500. eCollection 2025. Front Immunol. 2025. PMID: 40028333 Free PMC article. Review.
-
In vitro and in silico approaches manifest the anti-leishmanial activity of wild edible mushroom Amanita princeps.In Silico Pharmacol. 2024 Dec 24;13(1):6. doi: 10.1007/s40203-024-00287-0. eCollection 2025. In Silico Pharmacol. 2024. PMID: 39726904
References
-
- Beckham T.H., Lu P., Jones E.E., Marrison T., Lewis C.S., Cheng J.C., Ramshesh V.K., Beeson G., Beeson C.C., Drake R.R., et al. LCL124, a Cationic Analog of Ceramide, Selectively Induces Pancreatic Cancer Cell Death by Accumulating in Mitochondria. J. Pharmacol. Exp. Ther. 2013;344:167–178. doi: 10.1124/jpet.112.199216. - DOI - PMC - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources

