Is Insulin Receptor Substrate4 (IRS4) a Platform Involved in the Activation of Several Oncogenes?
- PMID: 37760618
- PMCID: PMC10526421
- DOI: 10.3390/cancers15184651
Is Insulin Receptor Substrate4 (IRS4) a Platform Involved in the Activation of Several Oncogenes?
Abstract
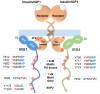
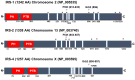
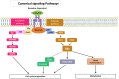
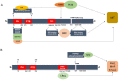
The IRS (insulin receptor substrate) family of scaffold proteins includes insulin receptor substrate-4 (IRS4), which is expressed only in a few cell lines, including human kidney, brain, liver, and thymus and some cell lines. Its N-terminus carries a phosphotyrosine-binding (PTB) domain and a pleckstrin homology domain (PH), which distinguishes it as a member of this family. In this paper, we collected data about the molecular mechanisms that explain the relevance of IRS4 in the development of cancer and identify IRS4 differences that distinguish it from IRS1 and IRS2. Search engines and different databases, such as PubMed, UniProt, ENSEMBL and SCANSITE 4.0, were used. We used the name of the protein that it encodes "(IRS-4 or IRS4)", or the combination of these terms with the word "(cancer)" or "(human)", for searches. Terms related to specific tumor pathologies ("breast", "ovary", "colon", "lung", "lymphoma", etc.) were also used. Despite the lack of knowledge on IRS4, it has been reported that some cancers and benign tumors are characterized by high levels of IRS-4 expression. Specifically, the role of IRS-4 in different types of digestive tract neoplasms, gynecological tumors, lung cancers, melanomas, hematological tumors, and other less common types of cancers has been shown. IRS4 differs from IRS1 and IRS2 in that can activate several oncogenes that regulate the PI3K/Akt cascade, such as BRK and FER, which are characterized by tyrosine kinase-like activity without regulation via extracellular ligands. In addition, IRS4 can activate the CRKL oncogene, which is an adapter protein that regulates the MAP kinase cascade. Knowledge of the role played by IRS4 in cancers at the molecular level, specifically as a platform for oncogenes, may enable the identification and validation of new therapeutic targets.
Keywords: IRS4; MAPK pathway; PI3K/Akt pathway; cancer; translational biomarker.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Similar articles
-
Insulin receptor substrate-4 binds to Slingshot-1 phosphatase and promotes cofilin dephosphorylation.J Biol Chem. 2014 Sep 19;289(38):26302-26313. doi: 10.1074/jbc.M114.565945. Epub 2014 Aug 6. J Biol Chem. 2014. PMID: 25100728 Free PMC article.
-
FER-mediated phosphorylation and PIK3R2 recruitment on IRS4 promotes AKT activation and tumorigenesis in ovarian cancer cells.Elife. 2022 May 12;11:e76183. doi: 10.7554/eLife.76183. Elife. 2022. PMID: 35550247 Free PMC article.
-
IRS4 promotes the progression of non-small cell lung cancer and confers resistance to EGFR-TKI through the activation of PI3K/Akt and Ras-MAPK pathways.Exp Cell Res. 2021 Jun 15;403(2):112615. doi: 10.1016/j.yexcr.2021.112615. Epub 2021 Apr 21. Exp Cell Res. 2021. PMID: 33894221
-
Insulin Substrate Receptor (IRS) proteins in normal and malignant hematopoiesis.Clinics (Sao Paulo). 2018 Oct 11;73(suppl 1):e566s. doi: 10.6061/clinics/2018/e566s. Clinics (Sao Paulo). 2018. PMID: 30328953 Free PMC article. Review.
-
Multiple signal transduction pathways mediate interleukin-4-induced 3beta-hydroxysteroid dehydrogenase/Delta5-Delta4 isomerase in normal and tumoral target tissues.J Steroid Biochem Mol Biol. 2001 Jan-Mar;76(1-5):213-25. doi: 10.1016/s0960-0760(00)00148-5. J Steroid Biochem Mol Biol. 2001. PMID: 11384880 Review.
Cited by
-
Oocyte transcriptomes and follicular fluid proteomics of ovine atretic follicles reveal the underlying mechanisms of oocyte degeneration.BMC Genomics. 2025 Feb 1;26(1):97. doi: 10.1186/s12864-025-11291-9. BMC Genomics. 2025. PMID: 39893388 Free PMC article.
-
Prognosis value of circulating tumor cell PD‑L1 and baseline characteristics in patients with NSCLC treated with immune checkpoint inhibitors plus platinum‑containing drugs.Oncol Lett. 2024 Jan 30;27(3):131. doi: 10.3892/ol.2024.14264. eCollection 2024 Mar. Oncol Lett. 2024. PMID: 38362233 Free PMC article.
-
Identification of new tissue markers for the monitoring and standardization of penile cancer according to the degree of differentiation.Histol Histopathol. 2025 Jul;40(7):1013-1039. doi: 10.14670/HH-18-846. Epub 2024 Nov 7. Histol Histopathol. 2025. PMID: 39600260
-
Understanding the Role of Connexins in Hepatocellular Carcinoma: Molecular and Prognostic Implications.Cancers (Basel). 2024 Apr 17;16(8):1533. doi: 10.3390/cancers16081533. Cancers (Basel). 2024. PMID: 38672615 Free PMC article. Review.
-
Unraveling the Mystery of Insulin Resistance: From Principle Mechanistic Insights and Consequences to Therapeutic Interventions.Int J Mol Sci. 2025 Mar 19;26(6):2770. doi: 10.3390/ijms26062770. Int J Mol Sci. 2025. PMID: 40141412 Free PMC article. Review.
References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources

