Using Single-Case Experimental Design and Patient-Reported Outcome Measures to Evaluate the Treatment of Cancer-Related Cognitive Impairment in Clinical Practice
- PMID: 37760621
- PMCID: PMC10526413
- DOI: 10.3390/cancers15184643
Using Single-Case Experimental Design and Patient-Reported Outcome Measures to Evaluate the Treatment of Cancer-Related Cognitive Impairment in Clinical Practice
Abstract
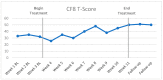
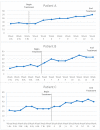
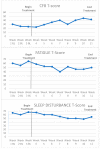
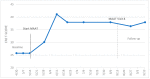
Cancer-related cognitive impairment (CRCI) affects a large proportion of cancer survivors and has significant negative effects on survivor function and quality of life (QOL). Treatments for CRCI are being developed and evaluated. Memory and attention adaptation training (MAAT) is a cognitive-behavioral therapy (CBT) demonstrated to improve CRCI symptoms and QOL in previous research. The aim of this article is to describe a single-case experimental design (SCED) approach to evaluate interventions for CRCI in clinical practice with patient-reported outcome measures (PROs). We illustrate the use of contemporary SCED methods as a means of evaluating MAAT, or any CRCI treatment, once clinically deployed. With the anticipated growth of cancer survivorship and concurrent growth in the number of survivors with CRCI, the treatment implementation and evaluation methods described here can be one way to assess and continually improve CRCI rehabilitative services.
Keywords: cancer survivorship; cognition; cognitive-behavioral therapy; single-case experimental design.
Conflict of interest statement
Ferguson is the lead author of
Figures

Similar articles
-
Cancer-related cognitive impairment: updates to treatment, the need for more evidence, and impact on quality of life-a narrative review.Ann Palliat Med. 2024 Sep;13(5):1265-1280. doi: 10.21037/apm-24-70. Epub 2024 Sep 9. Ann Palliat Med. 2024. PMID: 39260437 Review.
-
A randomized trial of videoconference-delivered cognitive behavioral therapy for survivors of breast cancer with self-reported cognitive dysfunction.Cancer. 2016 Jun 1;122(11):1782-91. doi: 10.1002/cncr.29891. Epub 2016 May 2. Cancer. 2016. PMID: 27135464 Clinical Trial.
-
Protocol for the Exercise, Cancer and Cognition - The ECCO-Study: A Randomized Controlled Trial of Simultaneous Exercise During Neo-/Adjuvant Chemotherapy in Breast Cancer Patients and Its Effects on Neurocognition.Front Neurol. 2022 Mar 25;13:777808. doi: 10.3389/fneur.2022.777808. eCollection 2022. Front Neurol. 2022. PMID: 35401389 Free PMC article.
-
Use of focused computerized cognitive training (Neuroflex) to improve symptoms in women with persistent chemotherapy-related cognitive impairment.Digit Health. 2023 Aug 13;9:20552076231192754. doi: 10.1177/20552076231192754. eCollection 2023 Jan-Dec. Digit Health. 2023. PMID: 37588161 Free PMC article.
-
Long-Term Cognitive Dysfunction in Cancer Survivors.Front Mol Biosci. 2021 Dec 14;8:770413. doi: 10.3389/fmolb.2021.770413. eCollection 2021. Front Mol Biosci. 2021. PMID: 34970595 Free PMC article. Review.
References
-
- Mandelblatt J.S., Small B.J., Luta G., Hurria A., Jim H., McDonald B.C., Graham D., Zhou X., Clapp J., Zhai W. Cancer-Related Cognitive Outcomes Among Older Breast Cancer Survivors in the Thinking and Living with Cancer Study. J. Clin. Oncol. 2018;36:3211–3222. doi: 10.1200/JCO.18.00140. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

