Horizontal Gene Transfer and Drug Resistance Involving Mycobacterium tuberculosis
- PMID: 37760664
- PMCID: PMC10526031
- DOI: 10.3390/antibiotics12091367
Horizontal Gene Transfer and Drug Resistance Involving Mycobacterium tuberculosis
Abstract
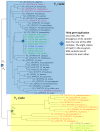
Mycobacterium tuberculosis (Mtb) acquires drug resistance at a rate comparable to that of bacterial pathogens that replicate much faster and have a higher mutation rate. One explanation for this rapid acquisition of drug resistance in Mtb is that drug resistance may evolve in other fast-replicating mycobacteria and then be transferred to Mtb through horizontal gene transfer (HGT). This paper aims to address three questions. First, does HGT occur between Mtb and other mycobacterial species? Second, what genes after HGT tend to survive in the recipient genome? Third, does HGT contribute to antibiotic resistance in Mtb? I present a conceptual framework for detecting HGT and analyze 39 ribosomal protein genes, 23S and 16S ribosomal RNA genes, as well as several genes targeted by antibiotics against Mtb, from 43 genomes representing all major groups within Mycobacterium. I also included mgtC and the insertion sequence IS6110 that were previously reported to be involved in HGT. The insertion sequence IS6110 shows clearly that the Mtb complex participates in HGT. However, the horizontal transferability of genes depends on gene function, as was previously hypothesized. HGT is not observed in functionally important genes such as ribosomal protein genes, rRNA genes, and other genes chosen as drug targets. This pattern can be explained by differential selection against functionally important and unimportant genes after HGT. Functionally unimportant genes such as IS6110 are not strongly selected against, so HGT events involving such genes are visible. For functionally important genes, a horizontally transferred diverged homologue from a different species may not work as well as the native counterpart, so the HGT event involving such genes is strongly selected against and eliminated, rendering them invisible to us. In short, while HGT involving the Mtb complex occurs, antibiotic resistance in the Mtb complex arose from mutations in those drug-targeted genes within the Mtb complex and was not gained through HGT.
Keywords: antibiotic resistance; horizontal gene transfer; isoniazid; phylogenetic incongruence; refampin.
Conflict of interest statement
The author declares no conflict of interest.
Figures




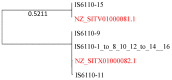
Similar articles
-
Comparative Genomic Analysis of Mycobacteriaceae Reveals Horizontal Gene Transfer-Mediated Evolution of the CRISPR-Cas System in the Mycobacterium tuberculosis Complex.mSystems. 2021 Jan 19;6(1):e00934-20. doi: 10.1128/mSystems.00934-20. mSystems. 2021. PMID: 33468705 Free PMC article.
-
ESX-1-Independent Horizontal Gene Transfer by Mycobacterium tuberculosis Complex Strains.mBio. 2021 May 18;12(3):e00965-21. doi: 10.1128/mBio.00965-21. mBio. 2021. PMID: 34006663 Free PMC article.
-
Horizontal Gene Transfer Building Prokaryote Genomes: Genes Related to Exchange Between Cell and Environment are Frequently Transferred.J Mol Evol. 2018 Apr;86(3-4):190-203. doi: 10.1007/s00239-018-9836-x. Epub 2018 Mar 19. J Mol Evol. 2018. PMID: 29556740
-
Horizontal gene transfer in cyanobacterial signature genes.Methods Mol Biol. 2009;532:339-66. doi: 10.1007/978-1-60327-853-9_20. Methods Mol Biol. 2009. PMID: 19271195 Review.
-
Horizontal transfer of antibiotic resistance genes in clinical environments.Can J Microbiol. 2019 Jan;65(1):34-44. doi: 10.1139/cjm-2018-0275. Epub 2018 Sep 24. Can J Microbiol. 2019. PMID: 30248271 Review.
Cited by
-
Unveiling Insights into the Whole Genome Sequencing of Mycobacterium spp. Isolated from Siamese Fighting Fish (Betta splendens).Animals (Basel). 2024 Oct 1;14(19):2833. doi: 10.3390/ani14192833. Animals (Basel). 2024. PMID: 39409782 Free PMC article.
-
Phylogeographic Analysis for Understanding Origin, Speciation, and Biogeographic Expansion of Invasive Asian Hornet, Vespa velutina Lepeletier, 1836 (Hymenoptera, Vespidae).Life (Basel). 2024 Oct 12;14(10):1293. doi: 10.3390/life14101293. Life (Basel). 2024. PMID: 39459593 Free PMC article.
-
Differential Selection for Translation Efficiency Shapes Translation Machineries in Bacterial Species.Microorganisms. 2024 Apr 10;12(4):768. doi: 10.3390/microorganisms12040768. Microorganisms. 2024. PMID: 38674712 Free PMC article.
-
Characterization of mycobacteria isolated from the Brazilian Atlantic Forest: a public health and bioprospection perspective.Front Microbiol. 2025 Apr 25;16:1558006. doi: 10.3389/fmicb.2025.1558006. eCollection 2025. Front Microbiol. 2025. PMID: 40351310 Free PMC article.
-
Evolution and emergence of Mycobacterium tuberculosis.FEMS Microbiol Rev. 2024 Mar 1;48(2):fuae006. doi: 10.1093/femsre/fuae006. FEMS Microbiol Rev. 2024. PMID: 38365982 Free PMC article. Review.
References
-
- Ford C.B., Lin P.L., Chase M.R., Shah R.R., Iartchouk O., Galagan J., Mohaideen N., Ioerger T.R., Sacchettini J.C., Lipsitch M., et al. Use of whole genome sequencing to estimate the mutation rate of Mycobacterium tuberculosis during latent infection. Nat. Genet. 2011;43:482–486. doi: 10.1038/ng.811. - DOI - PMC - PubMed
-
- Centers for Disease Control and Prevention Emergence of Mycobacterium tuberculosis with extensive resistance to second-line drugs–worldwide, 2000–2004. MMWR Morb. Mortal. Wkly. Rep. 2006;55:301–305. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

