Impact of Continuous Kidney Replacement Therapy and Hemoadsorption with CytoSorb on Antimicrobial Drug Removal in Critically Ill Children with Septic Shock: A Single-Center Prospective Study on a Pediatric Cohort
- PMID: 37760692
- PMCID: PMC10525765
- DOI: 10.3390/antibiotics12091395
Impact of Continuous Kidney Replacement Therapy and Hemoadsorption with CytoSorb on Antimicrobial Drug Removal in Critically Ill Children with Septic Shock: A Single-Center Prospective Study on a Pediatric Cohort
Abstract
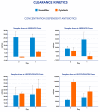
Background: Extracorporeal therapies (ET) are increasingly used in pediatric settings as adjuvant therapeutic strategies for overwhelming inflammatory conditions. Although these treatments seem to be effective for removing inflammatory mediators, their influence on antimicrobials pharmacokinetic should not be neglected. Methods: A prospective observational study of children admitted to the pediatric intensive care unit (PICU) with a diagnosis of sepsis/septic shock. All critically ill children received hemoadsorption treatment with CytoSorb (CS) in combination with CKRT. Therapeutic drug monitoring has been performed on 10 critically ill children, testing four antimicrobial molecules: meropenem, ceftazidime, amikacin and levofloxacin. In order to evaluate the total and isolated CKRT and CS contributions to antibiotic removal, blood samples at each circuit point (post-hemofilter, post-CS and in the effluent line) were performed. Therefore, the clearance and mass Removal (MR) of the hemofilter and CS were calculated. Results: Our preliminary report describes a different impact of CS on these target drugs removal: CS clearance was low for amikacine (6-12%), moderate for ceftazidime (43%) and moderate to high for levofloxacine (52-72%). Higher MR and clearance were observed with CKRT compared to CS. To the best of our knowledge, this is the first report regarding pharmacokinetic dynamics in critically ill children treated with CKRT and CS for septic shock.
Keywords: CKRT; CytoSorb; antimicrobials; clearance; extracorporeal therapies; hemoadsorption; mass removal; therapeutic drug monitoring (TDM).
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


Similar articles
-
Impact of hemoadsorption with CytoSorb® on meropenem and piperacillin exposure in critically ill patients in a post-CKRT setup: a single-center, retrospective data analysis.Intensive Care Med Exp. 2025 Jan 18;13(1):7. doi: 10.1186/s40635-025-00716-0. Intensive Care Med Exp. 2025. PMID: 39826040 Free PMC article.
-
Hemoadsorption in the Management of Septic Shock: A Systematic Review and Meta-Analysis.J Clin Med. 2025 Mar 27;14(7):2285. doi: 10.3390/jcm14072285. J Clin Med. 2025. PMID: 40217734 Free PMC article. Review.
-
Role of Hemoperfusion With CytoSorb Associated With Continuous Kidney Replacement Therapy on Renal Outcome in Critically III Children With Septic Shock.Front Pediatr. 2021 Aug 24;9:718049. doi: 10.3389/fped.2021.718049. eCollection 2021. Front Pediatr. 2021. PMID: 34504817 Free PMC article.
-
Impact of CytoSorb and CKRT on hemodynamics in pediatric patients with septic shock: the PedCyto study.Front Pediatr. 2023 Sep 15;11:1259384. doi: 10.3389/fped.2023.1259384. eCollection 2023. Front Pediatr. 2023. PMID: 37780052 Free PMC article.
-
Adjunctive Hemoadsorption Therapy with CytoSorb in Patients with Septic/Vasoplegic Shock: A Best Practice Consensus Statement.J Clin Med. 2023 Nov 21;12(23):7199. doi: 10.3390/jcm12237199. J Clin Med. 2023. PMID: 38068250 Free PMC article. Review.
Cited by
-
Impact of hemoadsorption with CytoSorb® on meropenem and piperacillin exposure in critically ill patients in a post-CKRT setup: a single-center, retrospective data analysis.Intensive Care Med Exp. 2025 Jan 18;13(1):7. doi: 10.1186/s40635-025-00716-0. Intensive Care Med Exp. 2025. PMID: 39826040 Free PMC article.
-
Pharmacokinetic/pharmacodynamic issues for optimizing treatment with beta-lactams of Gram-negative infections in critically ill orthotopic liver transplant recipients: a comprehensive review.Front Antibiot. 2024 Jun 17;3:1426753. doi: 10.3389/frabi.2024.1426753. eCollection 2024. Front Antibiot. 2024. PMID: 39816245 Free PMC article. Review.
-
Use of extracorporeal blood purification therapies in sepsis: the current paradigm, available evidence, and future perspectives.Crit Care. 2024 Dec 25;28(1):432. doi: 10.1186/s13054-024-05220-7. Crit Care. 2024. PMID: 39722012 Free PMC article. Review.
-
Hemoadsorption in the Management of Septic Shock: A Systematic Review and Meta-Analysis.J Clin Med. 2025 Mar 27;14(7):2285. doi: 10.3390/jcm14072285. J Clin Med. 2025. PMID: 40217734 Free PMC article. Review.
References
-
- Hoff B.M., Maker J.H., Dager W.E., Heintz B.H. Antibiotic dosing for critically ill adult patients receiving intermittent hemodialysis, prolonged intermittent renal replacement therapy, and continuous renal replacement therapy: An update. Ann. Pharmacother. 2020;54:43–55. doi: 10.1177/1060028019865873. - DOI - PubMed
LinkOut - more resources
Full Text Sources

